Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein
- PMID: 16428458
- PMCID: PMC1347045
- DOI: 10.1128/MCB.26.3.1063-1076.2006
Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein
Abstract
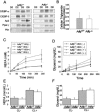
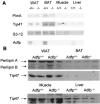
Adipose differentiation-related protein (ADFP; also known as ADRP or adipophilin), is a lipid droplet (LD) protein found in most cells and tissues. ADFP expression is strongly induced in cells with increased lipid load. We have inactivated the Adfp gene in mice to better understand its role in lipid accumulation. The Adfp-deficient mice have unaltered adipose differentiation or lipolysis in vitro or in vivo. Importantly, they display a 60% reduction in hepatic triglyceride (TG) and are resistant to diet-induced fatty liver. To determine the mechanism for the reduced hepatic TG content, we measured hepatic lipogenesis, very-low-density lipoprotein (VLDL) secretion, and lipid uptake and utilization, all of which parameters were shown to be similar between mutant and wild-type mice. The finding of similar VLDL output in the presence of a reduction in total TG in the Adfp-deficient liver is explained by the retention of TG in the microsomes where VLDL is assembled. Given that lipid droplets are thought to form from the outer leaflet of the microsomal membrane, the reduction of TG in the cytosol with concomitant accumulation of TG in the microsome of Adfp-/- cells suggests that ADFP may facilitate the formation of new LDs. In the absence of ADFP, impairment of LD formation is associated with the accumulation of microsomal TG but a reduction in TG in other subcellular compartments.
Figures



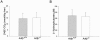



References
-
- Bildirici, I., C. R. Roh, W. T. Schaiff, B. M. Lewkowski, D. M. Nelson, and Y. Sadovsky. 2003. The lipid droplet-associated protein adipophilin is expressed in human trophoblasts and is regulated by peroxisomal proliferator-activated receptor-gamma/retinoid X receptor. J. Clin. Endocrinol. Metab. 88:6056-6062. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
-
- Brasaemle, D. L., T. Barber, N. E. Wolins, G. Serrero, E. J. Blanchette-Mackie, and C. Londos. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38:2249-2263. - PubMed
-
- Brasaemle, D. L., G. Dolios, L. Shapiro, and R. Wang. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279:46835-46842. - PubMed
-
- Buechler, C., M. Ritter, C. Q. Duong, E. Orso, M. Kapinsky, and G. Schmitz. 2001. Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim. Biophys. Acta 1532:97-104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
