Respiratory failure due to differentiation arrest and expansion of alveolar cells following lung-specific loss of the transcription factor C/EBPalpha in mice
- PMID: 16428462
- PMCID: PMC1347037
- DOI: 10.1128/MCB.26.3.1109-1123.2006
Respiratory failure due to differentiation arrest and expansion of alveolar cells following lung-specific loss of the transcription factor C/EBPalpha in mice
Abstract
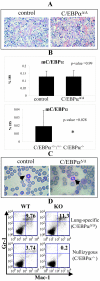
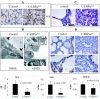
The leucine zipper family transcription factor CCAAT enhancer binding protein alpha (C/EBPalpha) inhibits proliferation and promotes differentiation in various cell types. In this study, we show, using a lung-specific conditional mouse model of C/EBPalpha deletion, that loss of C/EBPalpha in the respiratory epithelium leads to respiratory failure at birth due to an arrest in the type II alveolar cell differentiation program. This differentiation arrest results in the lack of type I alveolar cells and differentiated surfactant-secreting type II alveolar cells. In addition to showing a block in type II cell differentiation, the neonatal lungs display increased numbers of proliferating cells and decreased numbers of apoptotic cells, leading to epithelial expansion and loss of airspace. Consistent with the phenotype observed, genes associated with alveolar maturation, survival, and proliferation were differentially expressed. Taken together, these results identify C/EBPalpha as a master regulator of airway epithelial maturation and suggest that the loss of C/EBPalpha could also be an important event in the multistep process of lung tumorigenesis. Furthermore, this study indicates that exploring the C/EBPalpha pathway might have therapeutic benefits for patients with respiratory distress syndromes.
Figures







References
-
- Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57:289-300.
-
- Bennett, C. N., S. E. Ross, K. A. Longo, L. Bajnok, N. Hemati, K. W. Johnson, S. D. Harrison, and O. A. MacDougald. 2002. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277:30998-31004. - PubMed
-
- Bigelow, R. L., N. S. Chari, A. B. Unden, K. B. Spurgers, S. Lee, D. R. Roop, R. Toftgard, and T. J. McDonnell. 2004. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J. Biol. Chem. 279:1197-1205. - PubMed
-
- Bourbon, J. R., M. Rieutort, M. J. Engle, and P. M. Farrell. 1982. Utilization of glycogen for phospholipid synthesis in fetal rat lung. Biochim. Biophys. Acta 712:382-389. - PubMed
-
- Breathnach, O. S., B. Freidlin, B. Conley, M. R. Green, D. H. Johnson, D. R. Gandara, M. O'Connell, F. A. Shepherd, and B. E. Johnson. 2001. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J. Clin. Oncol. 19:1734-1742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
