Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression
- PMID: 16428725
- PMCID: PMC1360337
- DOI: 10.1128/IAI.74.2.830-838.2006
Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression
Abstract
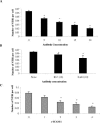
Nontypeable Haemophilus influenzae (NTHI) is an important respiratory pathogen. NTHI initiates infection by adhering to the airway epithelium. Here, we report that NTHI interacts with intracellular adhesion molecule 1 (ICAM-1) expressed by respiratory epithelial cells. A fourfold-higher number of NTHI bacteria adhered to Chinese hamster ovary (CHO) cells transfected with human ICAM-1 (CHO-ICAM-1) than to control CHO cells (P < or = 0.005). Blocking cell surface ICAM-1 with specific antibody reduced the adhesion of NTHI to A549 respiratory epithelial cells by 37% (P = 0.001) and to CHO-ICAM-1 cells by 69% (P = 0.005). Preincubating the bacteria with recombinant ICAM-1 reduced adhesion by 69% (P = 0.003). The adherence to CHO-ICAM-1 cells of NTHI strains deficient in the adhesins P5, P2, HMW1/2, and Hap or expressing a truncated lipooligosaccharide was compared to that of parental strains. Only strain 1128f-, which lacks the outer membrane protein (OMP) P5-homologous adhesin (P5 fimbriae), adhered less well than its parental strain. The numbers of NTHI cells adhering to CHO-ICAM-1 cells were reduced by 67% (P = 0.009) following preincubation with anti-P5 antisera. Furthermore, recombinant ICAM bound to an OMP preparation from strain 1128f+, which expresses P5, but not to that from its P5-deficient mutant, confirming a specific interaction between ICAM-1 and P5 fimbriae. Incubation of respiratory epithelial cells with NTHI increased ICAM-1 expression fourfold (P=0.001). Adhesion of NTHI to the respiratory epithelium, therefore, upregulates the expression of its own receptor. Blocking interactions between NTHI P5 fimbriae and ICAM-1 may reduce respiratory colonization by NTHI and limit the frequency and severity of NTHI infection.
Figures



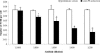


References
-
- Berendt, A. R., A. McDowall, A. G. Craig, P. A. Bates, M. J. Sternberg, K. Marsh, C. I. Newbold, and N. Hogg. 1992. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell 68:71-81. - PubMed
-
- Bochner, B. S., F. W. Luscinskas, M. A. Gimbrone, Jr., W. Newman, S. A. Sterbinsky, C. P. Derse-Anthony, D. Klunk, and R. P. Schleimer. 1991. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J. Exp. Med. 173:1553-1557. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

