Inhibition of TRIP1/S8/hSug1, a component of the human 19S proteasome, enhances mitotic apoptosis induced by spindle poisons
- PMID: 16432160
- PMCID: PMC1630635
- DOI: 10.1158/1535-7163.MCT-05-0126
Inhibition of TRIP1/S8/hSug1, a component of the human 19S proteasome, enhances mitotic apoptosis induced by spindle poisons
Abstract
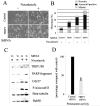
Mitotic spindle poisons (e.g., Taxol and vinblastine), used as chemotherapy drugs, inhibit mitotic spindle function, activate the mitotic spindle checkpoint, arrest cells in mitosis, and then cause cell death by mechanisms that are poorly understood. By expression cloning, we identified a truncated version of human TRIP1 (also known as S8, hSug1), an AAA (ATPases associated with diverse cellular activities) family ATPase subunit of the 19S proteasome regulatory complex, as an enhancer of spindle poison-mediated apoptosis. Stable expression of the truncated TRIP1/S8/hSug1 in HeLa cells [OP-TRIP1(88-406)] resulted in a decrease of measurable cellular proteasome activity, indicating that OP-TRIP1(88-406) had a dominant-negative effect on proteasome function. OP-TRIP1(88-406) revealed an increased apoptotic response after treatment with spindle poisons or with proteasome inhibitors. The increased apoptosis coincided with a significant decrease in expression of BubR1, a kinase required for activation and maintenance of the mitotic spindle checkpoint in response to treatment with spindle poisons. Small interfering RNA (siRNA)-mediated knockdown of TRIP1/S8/hSug1 resulted in a reduction of general proteasome activity and an increase in mitotic index. The siRNA treatment also caused increased cell death after spindle poison treatment. These results indicate that inhibition of TRIP1/S8/hSug1 function by expression of a truncated version of the protein or by siRNA-mediated suppression enhances cell death in response to spindle poison treatment. Current proteasome inhibitor drugs in trial as anticancer agents target elements of the 20S catalytic subcomplex. Our results suggest that targeting the ATPase subunits in 19S regulatory complex in the proteasome may enhance the antitumor effects of spindle poisons.
Figures




References
-
- Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–86. - PubMed
-
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. - PubMed
-
- Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;8:413–50. - PubMed
-
- Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int J Cancer. 1999;83(2):151–6. - PubMed
-
- Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

