Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase
- PMID: 16432180
- PMCID: PMC1360601
- DOI: 10.1073/pnas.0510772103
Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase
Abstract
Class I phosphoinositide 3-kinase contains four isoforms of the catalytic subunit, p110alpha, -beta, -gamma, and -delta. At physiological levels of expression, the wild-type p110alpha isoform lacks oncogenic potential, but gain-of-function mutations and overexpression of p110alpha are correlated with oncogenicity. The p110beta, -gamma, and -delta isoforms induce transformation of cultured cells as wild-type proteins. This oncogenic potential requires kinase activity and can be suppressed by the target of rapamycin inhibitor rapamycin. The p110delta isoform constitutively activates the Akt signaling pathway; p110gamma activates Akt only in the presence of serum. The isoforms differ in their requirements for upstream signaling. The transforming activity of the p110gamma isoform depends on rat sarcoma viral oncogene homolog (Ras) binding; preliminary data suggest the same for p110beta and indicate Ras-independent oncogenic potential of p110delta. The surprising oncogenic potential of the wild-type non-alpha isoforms of class I phosphoinositide 3-kinase may explain the dearth of cancer-specific mutations in these proteins, because these non-alpha isoforms could contribute to the oncogenic phenotype of the cell by differential expression.
Figures

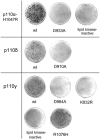

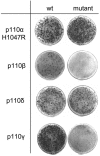
References
-
- Vanhaesebroeck, B., Leevers, S. J., Panayotou, G. & Waterfield, M. D. (1997) Trends Biochem. Sci. 22, 267–272. - PubMed
-
- Wymann, M. P., Zvelebil, M. & Laffargue, M. (2003) Trends Pharmacol. Sci. 24, 366–376. - PubMed
-
- Chantry, D., Vojtek, A., Kashishian, A., Holtzman, D. A., Wood, C., Gray, P. W., Cooper, J. A. & Hoekstra, M. F. (1997) J. Biol. Chem. 272, 19236–19241. - PubMed
-
- Okkenhaug, K. & Vanhaesebroeck, B. (2003) Nat. Rev. Immunol. 3, 317–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

