Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells
- PMID: 16432209
- PMCID: PMC1345707
- DOI: 10.1073/pnas.0508658103
Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells
Abstract
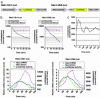
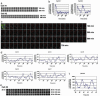
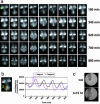
Notch signaling components such as the basic helix-loop-helix gene Hes1 are cyclically expressed by negative feedback in the presomitic mesoderm (PSM) and constitute the somite segmentation clock. Because Hes1 oscillation occurs in many cell types, this clock may regulate the timing in many biological systems. Although the Hes1 oscillator is stable in the PSM, it damps rapidly in other cells, suggesting that the oscillators in the former and the latter could be intrinsically different. Here, we have established the real-time bioluminescence imaging system of Hes1 expression and found that, although Hes1 oscillation is robust and stable in the PSM, it is unstable in the individual dissociated PSM cells, as in fibroblasts. Thus, the Hes1 oscillators in the individual PSM cells and fibroblasts are intrinsically similar, and these results, together with mathematical simulation, suggest that cell-cell communication is essential not only for synchronization but also for stabilization of cellular oscillators.
Figures


References
-
- Pourquié, O. (2003) Science 301, 328-330. - PubMed
-
- Bessho, Y. & Kageyama, R. (2003) Curr. Opin. Genet. Dev. 13, 1678-1690. - PubMed
-
- Weinmaster, G. & Kintner, C. (2003) Annu. Rev. Cell Dev. Biol. 19, 367-395. - PubMed
-
- Aulehla, A. & Herrmann, B. G. (2004) Genes Dev. 18, 2060-2067. - PubMed
-
- Giudicelli, F. & Lewis, J. (2004) Curr. Opin. Genet. Dev. 14, 407-414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

