The methyl-CpG binding protein MBD1 is required for PML-RARalpha function
- PMID: 16432238
- PMCID: PMC1360559
- DOI: 10.1073/pnas.0509343103
The methyl-CpG binding protein MBD1 is required for PML-RARalpha function
Abstract
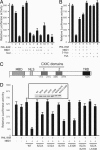
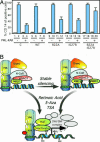
PML-RARalpha induces a block of hematopoietic differentiation and acute promyelocytic leukemia. This block is based on its capacity to inactivate target genes by recruiting histone deacetylase (HDAC) and DNA methyltransferase activities. Here we report that MBD1, a member of a conserved family of proteins able to bind methylated DNA, cooperates with PML-RARalpha in transcriptional repression and cellular transformation. PML-RARalpha recruits MBD1 to its target promoter through an HDAC3-mediated mechanism. Binding of HDAC3 and MBD1 is not confined to the promoter region but instead is spread over the locus. Knock-down of HDAC3 expression by RNA interference in acute promyelocytic leukemia cells alleviates PML-RAR-induced promoter silencing. We further demonstrate that retroviral expression of dominant-negative mutants of MBD1 in hematopoietic precursors compromises the ability of PML-RARalpha to block their differentiation and thus restored cell differentiation. Our results demonstrate that PML-RARalpha functions by recruiting an HDAC3-MBD1 complex that contributes to the establishment and maintenance of the silenced chromatin state.
Figures

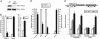
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

