Molecular determinants of the face map development in the trigeminal brainstem
- PMID: 16432893
- PMCID: PMC3556733
- DOI: 10.1002/ar.a.20285
Molecular determinants of the face map development in the trigeminal brainstem
Abstract
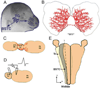
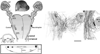
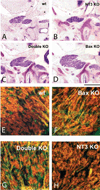
The perception of external sensory information by the brain requires highly ordered synaptic connectivity between peripheral sensory neurons and their targets in the central nervous system. Since the discovery of the whisker-related barrel patterns in the mouse cortex, the trigeminal system has become a favorite model for study of how its connectivity and somatotopic maps are established during development. The trigeminal brainstem nuclei are the first CNS regions where whisker-specific neural patterns are set up by the trigeminal afferents that innervate the whiskers. In particular, barrelette patterns in the principal sensory nucleus of the trigeminal nerve provide the template for similar patterns in the face representation areas of the thalamus and subsequently in the primary somatosensory cortex. Here, we describe and review studies of neurotrophins, multiple axon guidance molecules, transcription factors, and glutamate receptors during early development of trigeminal connections between the whiskers and the brainstem that lead to emergence of patterned face maps. Studies from our laboratories and others' showed that developing trigeminal ganglion cells and their axons depend on a variety of molecular signals that cooperatively direct them to proper peripheral and central targets and sculpt their synaptic terminal fields into patterns that replicate the organization of the whiskers on the muzzle. Similar mechanisms may also be used by trigeminothalamic and thalamocortical projections in establishing patterned neural modules upstream from the trigeminal brainstem.
Figures



Similar articles
-
Mapping the face in the somatosensory brainstem.Nat Rev Neurosci. 2010 Apr;11(4):252-63. doi: 10.1038/nrn2804. Epub 2010 Feb 24. Nat Rev Neurosci. 2010. PMID: 20179712 Free PMC article. Review.
-
Facial whisker pattern is not sufficient to instruct a whisker-related topographic map in the mouse somatosensory brainstem.Development. 2015 Nov 1;142(21):3704-12. doi: 10.1242/dev.128736. Epub 2015 Sep 28. Development. 2015. PMID: 26417040
-
Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats.J Neurophysiol. 1999 Nov;82(5):2765-75. doi: 10.1152/jn.1999.82.5.2765. J Neurophysiol. 1999. PMID: 10561443 Free PMC article.
-
Modelling the emergence of whisker barrels.Elife. 2020 Sep 29;9:e55588. doi: 10.7554/eLife.55588. Elife. 2020. PMID: 32988453 Free PMC article.
-
Barrelette map formation in the prenatal mouse brainstem.Curr Opin Neurobiol. 2018 Dec;53:210-219. doi: 10.1016/j.conb.2018.09.008. Epub 2018 Oct 17. Curr Opin Neurobiol. 2018. PMID: 30342228 Review.
Cited by
-
Mapping the face in the somatosensory brainstem.Nat Rev Neurosci. 2010 Apr;11(4):252-63. doi: 10.1038/nrn2804. Epub 2010 Feb 24. Nat Rev Neurosci. 2010. PMID: 20179712 Free PMC article. Review.
-
Alignment of EphA4 and ephrin-B2 expression patterns with developing modularity in the lateral cortex of the inferior colliculus.J Comp Neurol. 2018 Nov 1;526(16):2706-2721. doi: 10.1002/cne.24525. Epub 2018 Oct 22. J Comp Neurol. 2018. PMID: 30156295 Free PMC article.
-
Development of tactile sensory circuits in the CNS.Curr Opin Neurobiol. 2018 Dec;53:66-75. doi: 10.1016/j.conb.2018.06.001. Epub 2018 Jun 13. Curr Opin Neurobiol. 2018. PMID: 29908482 Free PMC article. Review.
-
A Brain-wide Circuit Model of Heat-Evoked Swimming Behavior in Larval Zebrafish.Neuron. 2018 May 16;98(4):817-831.e6. doi: 10.1016/j.neuron.2018.04.013. Epub 2018 May 3. Neuron. 2018. PMID: 29731253 Free PMC article.
-
Barrelettes without barrels in the American water shrew.PLoS One. 2013 Jun 3;8(6):e65975. doi: 10.1371/journal.pone.0065975. Print 2013. PLoS One. 2013. PMID: 23755296 Free PMC article.
References
-
- Altman J, Bayer SA. Development of the brainstem in the rat: IV, thymidine-radiographic study of the time of origin of neurons in the pontine region. J Comp Neurol. 1980;194:905–929. - PubMed
-
- Altman J, Bayer SA. The development of the rat spinal cord. Berlin: Springer; 1984. - PubMed
-
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. - PubMed
-
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger K, Leitner ML, Araki T, Johnson EM, Milbrandt J. Artemin, a novel member of the GDNF ligand family supports peripheral and central neurons and signals through the GFRα3-RET receptor complex. Neuron. 1998a;21:1291–1302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

