Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus
- PMID: 16432904
- PMCID: PMC3429851
- DOI: 10.1002/cne.20849
Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus
Abstract
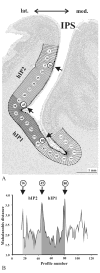
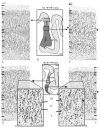
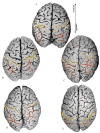
Anatomical studies in the macaque cortex and functional imaging studies in humans have demonstrated the existence of different cortical areas within the intraparietal sulcus (IPS). Such functional segregation, however, does not correlate with presently available architectonic maps of the human brain. This is particularly true for the classical Brodmann map, which is still widely used as an anatomical reference in functional imaging studies. The aim of this cytoarchitectonic mapping study was to use previously defined algorithms to determine whether consistent regions and borders can be found within the cortex of the anterior IPS in a population of 10 post-mortem human brains. Two areas, the human intraparietal area 1 (hIP1) and the human intraparietal area 2 (hIP2), were delineated in serial histological sections of the anterior, lateral bank of the human IPS. The region hIP1 is located posterior and medial to hIP2, and the former is always within the depths of the IPS. The latter, on the other hand, sometimes reaches the free surface of the superior parietal lobule. The delineations were registered to standard reference space, and probabilistic maps were calculated, thereby quantifying the intersubject variability in location and extent of both areas. In the future, they can be a tool for analyzing structure-function relationships and a basis for determining degrees of homology in the IPS among anthropoid primates. We conclude that the human IPS has a more finely grained parcellation than shown in Brodmann's map.
J. Comp. Neurol. 495:53-69, 2006. (c) 2006 Wiley-Liss, Inc.
Figures

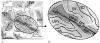






References
-
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. - PubMed
-
- Amunts K, Schleicher A, Ditterich A, Zilles K. Broca's region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. Erratum in: J Comp Neurol. 467:270. - PubMed
-
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink G, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space-The roles of Brodmann areas 44 and 45. NeuroImage. 2004;22:42–56. - PubMed
-
- Andersen RA, Brotchie PR, Mazzoni P. Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol. 1992;2:840–846. - PubMed
-
- Armstrong E, Zilles K, Schlaug G, Schleicher A. Comparative aspects of the primate posterior cingulate cortex. JCompNeurol. 1986;253:539–548. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

