A fluoroacetamidine-based inactivator of protein arginine deiminase 4: design, synthesis, and in vitro and in vivo evaluation
- PMID: 16433522
- PMCID: PMC1850713
- DOI: 10.1021/ja0576233
A fluoroacetamidine-based inactivator of protein arginine deiminase 4: design, synthesis, and in vitro and in vivo evaluation
Abstract
Protein arginine deiminase 4 (PAD4) is a calcium-dependent transcriptional corepressor that has been implicated in the onset and progression of rheumatoid arthritis. Herein we describe the synthesis and in vitro evaluation of a fluoroacetamidine-containing compound, N-alpha-benzoyl-N5-(2-fluoro-1-iminoethyl)-l-ornithine amide, 1, hereafter referred to as F-amidine, that is the most potent PAD4 inhibitor ever described. Additional studies described herein indicate that F-amidine can also inhibit PAD4 activity in vivo. The bioavailability of this compound suggests that F-amidine will be a powerful chemical probe of PAD4 function that can be used to dissect the roles of this enzyme in both rheumatoid arthritis and transcriptional control. The fact that inhibition is of an irreversible nature suggests that, with appropriate functionalization, F-amidine analogues will be robust activity-based protein-profiling and proteomic capture reagents.
Figures

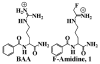


References
-
- Suzuki A, et al. Nat Genet. 2003;34:395–402. - PubMed
-
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. Bioessays. 2003;25:1106–1118. - PubMed
-
- Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. J Immunol. 2003;171:538–541. - PubMed
-
- Moscarello MA, Pritzker L, Mastronardi FG, Wood DD. J Neurochem. 2002;81:335–343. - PubMed
-
- Wang Y, et al. Science. 2004;306:279–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

