Localization of GRP78 to mitochondria under the unfolded protein response
- PMID: 16433633
- PMCID: PMC1450007
- DOI: 10.1042/BJ20051916
Localization of GRP78 to mitochondria under the unfolded protein response
Abstract
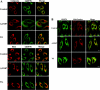
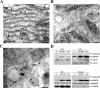
The ubiquitously expressed molecular chaperone GRP78 (78 kDa glucose-regulated protein) generally localizes to the ER (endoplasmic reticulum). GRP78 is specifically induced in cells under the UPR (unfolded protein response), which can be elicited by treatments with calcium ionophore A23187 and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase inhibitor TG (thapsigargin). By using confocal microscopy, we have demonstrated that GRP78 was concentrated in the perinuclear region and co-localized with the ER marker proteins, calnexin and PDI (protein disulphide-isomerase), in cells under normal growth conditions. However, treatments with A23187 and TG led to diminish its ER targeting, resulting in redirection into a cytoplasmic vesicular pattern, and overlapping with the mitochondrial marker MitoTracker. Cellular fractionation and protease digestion of isolated mitochondria from ER-stressed cells suggested that a significant portion of GRP78 is localized to the mitochondria and is protease-resistant. Localizations of GRP78 in ER and mitochondria were confirmed by using immunoelectron microscopy. In ER-stressed cells, GRP78 mainly localized within the mitochondria and decorated the mitochondrial membrane compartment. Submitochondrial fractionation studies indicated further that the mitochondria-resided GRP78 is mainly located in the intermembrane space, inner membrane and matrix, but is not associated with the outer membrane. Furthermore, radioactive labelling followed by subcellular fractionation showed that a significant portion of the newly synthesized GRP78 is localized to the mitochondria in cells under UPR. Taken together, our results indicate that, at least under certain circumstances, the ER-resided chaperone GRP78 can be retargeted to mitochondria and thereby may be involved in correlating UPR signalling between these two organelles.
Figures



References
-
- Brostrom M. A., Brostrom C. O. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. - PubMed
-
- Sitia R., Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature (London) 2003;426:891–894. - PubMed
-
- Berridge M. J. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. - PubMed
-
- Kaufman R. J., Scheuner D., Schroder M., Shen X., Lee K., Liu C. Y., Arnold S. M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002;3:411–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

