Effects of lipopolysaccharide-induced inflammation on expression of growth-associated genes by corticospinal neurons
- PMID: 16433912
- PMCID: PMC1403789
- DOI: 10.1186/1471-2202-7-8
Effects of lipopolysaccharide-induced inflammation on expression of growth-associated genes by corticospinal neurons
Abstract
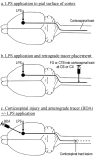
Background: Inflammation around cell bodies of primary sensory neurons and retinal ganglion cells enhances expression of neuronal growth-associated genes and stimulates axonal regeneration. We have asked if inflammation would have similar effects on corticospinal neurons, which normally show little response to spinal cord injury. Lipopolysaccharide (LPS) was applied onto the pial surface of the motor cortex of adult rats with or without concomitant injury of the corticospinal tract at C4. Inflammation around corticospinal tract cell bodies in the motor cortex was assessed by immunohistochemistry for OX42 (a microglia and macrophage marker). Expression of growth-associated genes c-jun, ATF3, SCG10 and GAP-43 was investigated by immunohistochemistry or in situ hybridisation.
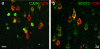
Results: Application of LPS induced a gradient of inflammation through the full depth of the motor cortex and promoted c-Jun and SCG10 expression for up to 2 weeks, and GAP-43 upregulation for 3 days by many corticospinal neurons, but had very limited effects on neuronal ATF3 expression. However, many glial cells in the subcortical white matter upregulated ATF3. LPS did not promote sprouting of anterogradely labelled corticospinal axons, which did not grow into or beyond a cervical lesion site.
Conclusion: Inflammation produced by topical application of LPS promoted increased expression of some growth-associated genes in the cell bodies of corticospinal neurons, but was insufficient to promote regeneration of the corticospinal tract.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

