Iron overload due to mutations in ferroportin
- PMID: 16434376
- PMCID: PMC3718253
Iron overload due to mutations in ferroportin
Abstract
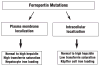
Iron overload disease due to mutations in ferroportin has a dominant inheritance and a variable clinical phenotype, such that some patients show early Küpffer cell iron loading and low transferrin saturation, while others show hepatocyte iron loading and high transferrin saturation. Studies expressing ferroportin mutant proteins in cultured cells have shown that mutant proteins fall into two main classes; proteins that do not localize to the cell surface and are unable to export iron, and those that localize to the cell surface but are unable to respond to the antimicrobial peptide hepcidin. Patients with mutant ferroportin proteins that do not localize to the cell surface show typical ferroportin disease with low transferrin saturation and early Küpffer cell iron loading, while patients with mutant proteins unable to respond to hepcidin show high transferrin saturation and early hepatocyte iron loading similar to classic hereditary hemochromatosis. The dominant genetic transmission of ferroportin-linked disorders is explained by the in vitro data, which suggest that ferroportin is a multimer and that the behavior of the mutant protein can affect the behavior of the wild type protein.
Figures

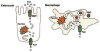
References
-
- Ganz T. Hepcidin - a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–82. - PubMed
-
- Loreal O, Haziza-Pigeon C, Troadec MB, Detivaud L, Turlin B, Courselaud B, et al. Hepcidin in iron metabolism. Curr Protein Pept Sci. 2005;6:279–91. - PubMed
-
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferro-portin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–81. - PubMed
-
- McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. - PubMed
-
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
