The redox couple of the cytochrome c cyanide complex: the contribution of heme iron ligation to the structural stability, chemical reactivity, and physiological behavior of horse cytochrome c
- PMID: 16434742
- PMCID: PMC2242453
- DOI: 10.1110/ps.051825906
The redox couple of the cytochrome c cyanide complex: the contribution of heme iron ligation to the structural stability, chemical reactivity, and physiological behavior of horse cytochrome c
Abstract
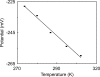
Contrary to most heme proteins, ferrous cytochrome c does not bind ligands such as cyanide and CO. In order to quantify this observation, the redox potential of the ferric/ferrous cytochrome c-cyanide redox couple was determined for the first time by cyclic voltammetry. Its E0' was -240 mV versus SHE, equivalent to -23.2 kJ/mol. The entropy of reaction for the reduction of the cyanide complex was also determined. From a thermodynamic cycle that included this new value for the cyt c cyanide complex E0', the binding constant of cyanide to the reduced protein was estimated to be 4.7 x 10(-3) L M(-1) or 13.4 kJ/mol (3.2 kcal/mol), which is 48.1 kJ/mol (11.5 kcal/mol) less favorable than the binding of cyanide to ferricytochrome c. For coordination of cyanide to ferrocytochrome c, the entropy change was earlier experimentally evaluated as 92.4 J mol(-1) K(-1) (22.1 e.u.) at 25 K, and the enthalpy change for the same net reaction was calculated to be 41.0 kJ/mol (9.8 kcal/mol). By taking these results into account, it was discovered that the major obstacle to cyanide coordination to ferrocytochrome c is enthalpic, due to the greater compactness of the reduced molecule or, alternatively, to a lower rate of conformational fluctuation caused by solvation, electrostatic, and structural factors. The biophysical consequences of the large difference in the stabilities of the closed crevice structures are discussed.
Figures


References
-
- Balducci, G. and Costa, G. 1993. The four-member square scheme in cyclic voltammetry: General solution for Nerstian electron transfers. J. Electroanal. Chem. 348: 355–365.
-
- Bard, A.J. and Faulkner, L.R. 2001. Electrochemical methods: Fundamentals and applications. Wiley & Sons, Inc., New York.
-
- Bertrand, P., Mbarki, O., Asso, M., Blanchard, L., Guerlesquin, F., and Tegoni, M. 1995. Control of the redox potential in c-type cytochromes: Importance of the entropic contribution. Biochemistry 34: 11071–11079. - PubMed
-
- Brautigan, D.L., Ferguson-Miller, S., and Margoliash, E. 1978. Mitochondrial cytochrome c: Preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 53: 128–164. - PubMed
-
- Brayer, G.D. and Murphy, M.E.P. 1996. Structural studies of eukaryotic cytochromes c. In Cytochrome c—A multidisciplinary approach (eds. R.A. Scott and A. Grant Mauk), pp. 103–166. University Science Books, Sausalito, CA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

