S-Adenosylhomocysteine hydrolase deficiency: a second patient, the younger brother of the index patient, and outcomes during therapy
- PMID: 16435181
- PMCID: PMC2441944
- DOI: 10.1007/s10545-005-0192-9
S-Adenosylhomocysteine hydrolase deficiency: a second patient, the younger brother of the index patient, and outcomes during therapy
Abstract
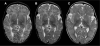
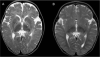
S-Adenosylhomocysteine (AdoHcy) hydrolase deficiency has been proven in a human only once, in a recently described Croatian boy. Here we report the clinical course and biochemical abnormalities of the younger brother of this proband. This younger brother has the same two mutations in the gene encoding AdoHcy hydrolase, and has been monitored since birth. We report, as well, outcomes during therapy for both patients. The information obtained suggests that the disease starts in utero and is characterized primarily by neuromuscular symptomatology (hypotonia, sluggishness, psychomotor delay, absent tendon reflexes, delayed myelination). The laboratory abnormalities are markedly increased creatine kinase and elevated aminotransferases, as well as specific amino acid aberrations that pinpoint the aetiology. The latter include, most importantly, markedly elevated plasma AdoHcy. Plasma S-adenosylmethionine (AdoMet) is also elevated, as is methionine (although the hypermethioninaemia may be absent or nonsignificant in the first weeks of life). The disease seems to be at least to some extent treatable, as shown by improved myelination and psychomotor development during dietary methionine restriction and supplementation with creatine and phosphatidylcholine.
Figures




References
-
- Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism. 1993;42:1448–1460. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brosnan JT, Jacobs RL, Stead LM, Brosnan ME. Methylation demand: a key determinant of homocysteine metabolism. Acta Biochimica Polonica. 2004;51:405–413. - PubMed
-
- Capdevila A, Wagner C. Measurement of plasma S-adenosylmethionine and S-adenosylhomocysteine as their fluorescent isoindoles. Analyt Biochem. 1998;264:180–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

