Regulation of fibroblast growth factor-23 signaling by klotho
- PMID: 16436388
- PMCID: PMC2637204
- DOI: 10.1074/jbc.C500457200
Regulation of fibroblast growth factor-23 signaling by klotho
Abstract
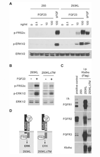
The aging suppressor gene Klotho encodes a single-pass transmembrane protein. Klotho-deficient mice exhibit a variety of aging-like phenotypes, many of which are similar to those observed in fibroblast growth factor-23 (FGF23)-deficient mice. To test the possibility that Klotho and FGF23 may function in a common signal transduction pathway(s), we investigated whether Klotho is involved in FGF signaling. Here we show that Klotho protein directly binds to multiple FGF receptors (FGFRs). The Klotho-FGFR complex binds to FGF23 with higher affinity than FGFR or Klotho alone. In addition, Klotho significantly enhanced the ability of FGF23 to induce phosphorylation of FGF receptor substrate and ERK in various types of cells. Thus, Klotho functions as a cofactor essential for activation of FGF signaling by FGF23.
Figures



References
-
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y. Nature. 1997;390:45–51. - PubMed
-
- Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Biochem. Biophys. Res. Commun. 2006;339:827–832. - PubMed
-
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. EBS Lett. 2004;565:143–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

