Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus
- PMID: 16436502
- PMCID: PMC1413659
- DOI: 10.1073/pnas.0510598103
Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus
Abstract
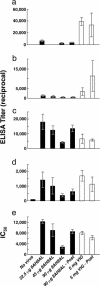
Chimpanzee Fabs against the B5 envelope glycoprotein of vaccinia virus were isolated and converted into complete mAbs with human gamma 1 heavy chain constant regions. The two mAbs (8AH8AL and 8AH7AL) displayed high binding affinities to B5 (Kd of 0.2 and 0.7 nM). The mAb 8AH8AL inhibited the spread of vaccinia virus as well as variola virus (the causative agent of smallpox) in vitro, protected mice from subsequent intranasal challenge with virulent vaccinia virus, protected mice when administered 2 days after challenge, and provided significantly greater protection than that afforded by a previously isolated rat anti-B5 mAb (19C2) or by vaccinia immune globulin. The mAb bound to a conformational epitope between amino acids 20 and 130 of B5. These chimpanzee/human anti-B5 mAbs may be useful in the prevention and treatment of vaccinia virus-induced complications of vaccination against smallpox and may also be effective in the immunoprophylaxis and immunotherapy of smallpox.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures




References
-
- Henderson D. A. Science. 1999;283:1279–1282. - PubMed
-
- Fulginiti V. A., Papier A., Lane J. M., Neff J. M., Henderson D. A. Clin. Infect. Dis. 2003;37:251–271. - PubMed
-
- Kempe C. H. Pediatrics. 1960;26:176–189. - PubMed
-
- Feery B. J. Vox Sang. 1976;31:68–76. - PubMed
-
- Hopkins R. J., Kramer W. G., Blackwelder W. C., Ashtekar M., Hague L., Winker-La Roche S. D., Berezuk G., Smith D., Leese P. T. Clin. Infect. Dis. 2004;39:759–766. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

