Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors
- PMID: 16436596
- PMCID: PMC6674560
- DOI: 10.1523/JNEUROSCI.3970-05.2006
Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors
Abstract
The role of multipotential progenitors and neural stem cells in the adult subventricular zone (SVZ) as cell-of-origin of glioblastoma has been suggested by studies on human tumors and transgenic mice. However, it is still unknown whether glial tumors are generated by all of the heterogeneous SVZ cell types or only by specific subpopulations of cells. It has been proposed that transformation could result from lack of apoptosis and increased self-renewal, but the definition of the properties leading to neoplastic transformation of SVZ cells are still elusive. This study addresses these questions in mice carrying the deletion of p53, a tumor-suppressor gene expressed in the SVZ. We show here that, although loss of p53 by itself is not sufficient for tumor formation, it provides a proliferative advantage to the slow- and fast-proliferating subventricular zone (SVZ) populations associated with their rapid differentiation. This results in areas of increased cell density that are distributed along the walls of the lateral ventricles and often associated with increased p53-independent apoptosis. Transformation occurs when loss of p53 is associated with a mutagenic stimulus and is characterized by dramatic changes in the properties of the quiescent adult SVZ cells, including enhanced self-renewal, recruitment to the fast-proliferating compartment, and impaired differentiation. Together, these findings provide a cellular mechanism for how the slow-proliferating SVZ cells can give rise to glial tumors in the adult brain.
Figures


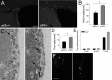
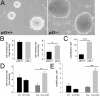
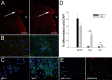



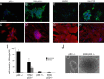
References
-
- Alvarez-Buylla A, Lim DA (2004). For the long run: maintaining germinal niches in the adult brain. Neuron 41:683–686. - PubMed
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA (2002). Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1:269–277. - PubMed
-
- Berger F, Gay E, Pelletier L, Tropel P, Wion D (2004). Development of gliomas: potential role of asymmetrical cell division of neural stem cells. Lancet Oncol 5:511–514. - PubMed
-
- Bouvier C, Bartoli C, Aguirre-Cruz L, Virard I, Colin C, Fernandez C, Gouvernet J, Figarella-Branger D (2003). Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J Neurosurg 99:344–350. - PubMed
-
- Chow BM, Li YQ, Wong CS (2000). Radiation-induced apoptosis in the adult central nervous system is p53-dependent. Cell Death Differ 7:712–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
