Peripheral myelin protein 22 is in complex with alpha6beta4 integrin, and its absence alters the Schwann cell basal lamina
- PMID: 16436605
- PMCID: PMC6674566
- DOI: 10.1523/JNEUROSCI.2618-05.2006
Peripheral myelin protein 22 is in complex with alpha6beta4 integrin, and its absence alters the Schwann cell basal lamina
Abstract
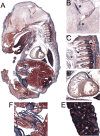
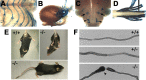
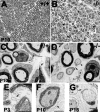
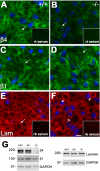
Peripheral myelin protein 22 (PMP22) is a tetraspan membrane glycoprotein, the misexpression of which is associated with hereditary demyelinating neuropathies. Myelinating Schwann cells (SCs) produce the highest levels of PMP22, yet the function of the protein in peripheral nerve biology is unresolved. To investigate the potential roles of PMP22, we engineered a novel knock-out (-/-) mouse line by replacing the first two coding exons of pmp22 with the lacZ reporter. PMP22-deficient mice show strong beta-galactosidase reactivity in peripheral nerves, cartilage, intestines, and lungs, whereas phenotypically they display the characteristics of tomaculous neuropathy. In the absence of PMP22, myelination of peripheral nerves is delayed, and numerous axon-SC profiles show loose basal lamina, suggesting altered interactions of the glial cells with the extracellular matrix. The levels of beta4 integrin, a molecule involved in the linkage between SCs and the basal lamina, are severely reduced in nerves of PMP22-deficient mice. During early stages of myelination, PMP22 and beta4 integrin are coexpressed at the cell surface and can be coimmunoprecipitated together with laminin and alpha6 integrin. In agreement, in clone A colonic carcinoma cells, epitope-tagged PMP22 forms a complex with beta4 integrin. Together, these data indicate that PMP22 is a binding partner in the integrin/laminin complex and is involved in mediating the interaction of SCs with the extracellular environment.
Figures

References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U (1995). Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat Genet 3:274–280. - PubMed
-
- Baechner D, Liehr T, Hameister H, Altenberger H, Grehl H, Suter U, Rautenstrauss B (1995). Widespread expression of the peripheral myelin protein-22 gene (PMP22) in neural and non-neural tissues during murine development. J Neurosci Res 42:733–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
