Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse
- PMID: 16436610
- PMCID: PMC6674564
- DOI: 10.1523/JNEUROSCI.4237-05.2006
Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse
Abstract
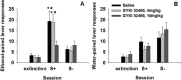
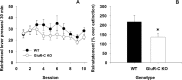
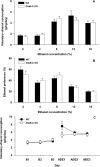
Craving and relapse are core symptoms of drug addiction and alcoholism. It is suggested that, after chronic drug consumption, long-lasting neuroplastic changes within the glutamatergic system are important determinants of addictive behavior. Here, we show that the AMPA type glutamate receptor plays a crucial role in alcohol craving and relapse. We observed, in two animal models of alcohol craving and relapse, that the AMPA antagonist GYKI 52466 [1-(4-aminophenyl)-4-methyl-7, 8-methylenedioxy-5H-2, 3-benzodiazepine] dose-dependently reduced cue-induced reinstatement of alcohol-seeking behavior and the alcohol deprivation effect. The involvement of the AMPA receptor in these phenomena was further studied using mice deficient for the GluR-C AMPA subunit [GluR-C knock-out (KO)]. GluR-C KOs displayed a blunted, cue-induced reinstatement response and alcohol deprivation effect, when compared with wild-type controls; however, no differences between genotypes could be observed regarding ethanol self-administration under operant or home cage drinking conditions. These results imply a role for GluR-C in alcohol relapse, although this phenotype could also be attributable to a reduction in the total number of AMPA receptors in specific brain areas. In conclusion, AMPA receptors seem to be involved in the neuroplastic changes underlying alcohol seeking behavior and relapse. Thus, AMPA receptors represent a novel therapeutic target in preventing relapse.
Figures


References
-
- Bachteler D, Spanagel R (2005). Glutamatergic compounds: preclinical data. In: Drugs for relapse prevention (Spanagel R, Mann KF, eds) pp. 205–216. Basel: Birkhäuser.
-
- Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R (2005). The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology 30:1104–1110. - PubMed
-
- Bäckstrom P, Hyytiä P (2004). Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res 28:558–565. - PubMed
-
- Bäckstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R (2004). mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 29:921–928. - PubMed
-
- Broadbent J, Kampmueller KM, Koonse SA (2003). Expression of behavioural sensitization to ethanol in DBA/2J mice: The role of NMDA and non-NMDA glutamate receptors. Psychopharmacology 167:225–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
