Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage
- PMID: 16436679
- PMCID: PMC1606508
- DOI: 10.2353/ajpath.2006.050599
Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage
Abstract
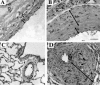
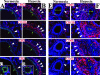
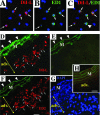
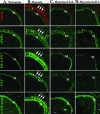
Vascular remodeling in chronic hypoxic pulmonary hypertension includes marked fibroproliferative changes in the pulmonary artery (PA) adventitia. Although resident PA fibroblasts have long been considered the primary contributors to these processes, we tested the hypothesis that hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage, termed fibrocytes. Using two neonatal animal models (rats and calves) of chronic hypoxic pulmonary hypertension, we demonstrated a dramatic perivascular accumulation of mononuclear cells of a monocyte/macrophage lineage (expressing CD45, CD11b, CD14, CD68, ED1, ED2). Many of these cells produced type I collagen, expressed alpha-smooth muscle actin, and proliferated, thus exhibiting mesenchymal cell characteristics attributed to fibrocytes. The blood-borne origin of these cells was confirmed in experiments wherein circulating monocytes/macrophages of chronically hypoxic rats were in vivo-labeled with DiI fluorochrome via liposome delivery and subsequently identified in the remodeled pulmonary, but not systemic, arterial adventitia. The DiI-labeled cells that appeared in the vessel wall expressed monocyte/macrophage markers and procollagen. Selective depletion of this monocytic cell population, using either clodronate-liposomes or gadolinium chloride, prevented pulmonary adventitial remodeling (ie, production of collagen, fibronectin, and tenascin-C and accumulation of myofibroblasts). We conclude that circulating mesenchymal precursors of a monocyte/macrophage lineage, including fibrocytes, are essential contributors to hypoxia-induced pulmonary vascular remodeling.
Figures





References
-
- Stenmark KR, Mecham R. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. - PubMed
-
- Jeffery T, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis. 2002;45:173–202. - PubMed
-
- Rabinovitch M. Pulmonary vascular remodeling in hypoxic pulmonary hypertension. Yuan JX-J, editor. Dordrecht: Kluwer Academic Publishers,; Hypoxic Pulmonary VasoconstrictionCellular and Molecular Mechanisms. 2004:pp 403–418.
-
- Meyrick B. The pathology of pulmonary artery hypertension. Clin Chest Med. 2001;22:393–404. - PubMed
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl S):13S–24S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

