Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury
- PMID: 16436682
- PMCID: PMC1780159
- DOI: 10.2353/ajpath.2006.050759
Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury
Abstract
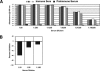
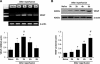
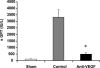
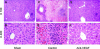
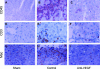
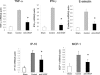
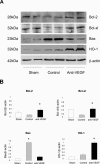
Although hypoxia stimulates the expression of vascular endothelial growth factor (VEGF), little is known of the role or mechanism by which VEGF functions after ischemia and reperfusion (I/R) injury. In this report, we first evaluated the expression of VEGF in a mouse model of liver warm ischemia. We found that the expression of VEGF increased after ischemia but peaked between 2 and 6 hours after reperfusion. Mice were treated with a neutralizing anti-mouse VEGF antiserum (anti-VEGF) or control serum daily from day -1 (1 day before the initiation of ischemia). Treatment with anti-VEGF significantly reduced serum glutaminic pyruvic transaminase levels and reduced histological evidence of hepatocellular damage compared with controls. Anti-VEGF also markedly decreased T-cell, macrophage, and neutrophil accumulation within livers and reduced the frequency of intrahepatic apoptotic terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling-positive cells. Moreover, there was a reduction in the expression of pro-inflammatory cytokines (tumor necrosis factor-alpha and interferon-gamma), chemokines (interferon-inducible protein-10 and monocyte chemoattractant protein-1) and adhesion molecules (E-selectin) in parallel with enhanced expression of anti-apoptotic genes (Bcl-2/Bcl-xl and heme oxygenase-1) in anti-VEGF-treated animals. In conclusion, hypoxia-inducible VEGF expression by hepatocytes modulates leukocyte trafficking and leukocyte-induced injury in a mouse liver model of warm I/R injury, demonstrating the importance of endogenous VEGF production in the pathophysiology of hepatic I/R injury.
Figures
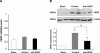


References
-
- Farmer DG, Amersi F, Busuttil RW, Kupiec-Weglinski JW. Current concepts in ischemia and reperfusion injury in the liver. Transplant Rev. 2000;14:106–126.
-
- Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury: a fresh look. Exp Mol Pathol. 2003;74:86–93. - PubMed
-
- Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. - PubMed
-
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

