In vitro antiretroviral activity and in vitro toxicity profile of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor for treatment of human immunodeficiency virus infection
- PMID: 16436719
- PMCID: PMC1366874
- DOI: 10.1128/AAC.50.2.625-631.2006
In vitro antiretroviral activity and in vitro toxicity profile of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor for treatment of human immunodeficiency virus infection
Abstract
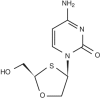
SPD754 (AVX754) is a deoxycytidine analogue nucleotide reverse transcriptase inhibitor (NRTI) in clinical development. These studies characterized the in vitro activity of SPD754 against NRTI-resistant human immunodeficiency virus type 1 (HIV-1) and non-clade B HIV-1 isolates, its activity in combination with other antiretrovirals, and its potential myelotoxicity and mitochondrial toxicity. SPD754 was tested against 50 clinical HIV-1 isolates (5 wild-type isolates and 45 NRTI-resistant isolates) in MT-4 cells using the Antivirogram assay. SPD754 susceptibility was reduced 1.2- to 2.2-fold against isolates resistant to zidovudine (M41L, T215Y/F, plus a median of three additional nucleoside analogue mutations [NAMs]) and/or lamivudine (M184V) and was reduced 1.3- to 2.8-fold against isolates resistant to abacavir (L74V, Y115F, and M184V plus one other NAM) or stavudine (V75T/M, M41L, T215F/Y, and four other NAMs). Insertions at amino acid position 69 and Q151M mutations (with or without M184V) reduced SPD754 susceptibility 5.2-fold and 14- to 16-fold, respectively (these changes gave values comparable to or less than the corresponding values for zidovudine, lamivudine, abacavir, and didanosine). SPD754 showed similar activity against isolates of group M HIV-1 clades, including A/G, B, C, D, A(E), D/F, F, and H. SPD754 showed additive effects in combination with other NRTIs, tenofovir, nevirapine, or saquinavir. SPD754 had no significant effects on cell viability or mitochondrial DNA in HepG2 or MT-4 cells during 28-day exposure at concentrations up to 200 microM. SPD754 showed a low potential for myelotoxicity against human bone marrow. In vitro, SPD754 retained activity against most NRTI-resistant HIV-1 clinical isolates and showed a low propensity to cause myelotoxicity and mitochondrial toxicity.
Figures
Similar articles
-
In vitro activity of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor (NRTI), against 215 HIV-1 isolates resistant to other NRTIs.Antivir Chem Chemother. 2005;16(5):295-302. doi: 10.1177/095632020501600502. Antivir Chem Chemother. 2005. PMID: 16245645
-
Prevalence, genotypic associations and phenotypic characterization of K65R, L74V and other HIV-1 RT resistance mutations in a commercial database.Antivir Ther. 2008;13(2):189-97. Antivir Ther. 2008. PMID: 18505170
-
Mutational patterns in the HIV genome and cross-resistance following nucleoside and nucleotide analogue drug exposure.Antivir Ther. 2001;6 Suppl 3:25-44. Antivir Ther. 2001. PMID: 11678471 Review.
-
Drug resistance and drug combination features of the human immunodeficiency virus inhibitor, BCH-10652 [(+/-)-2'-deoxy-3'-oxa-4'-thiocytidine, dOTC].Antivir Chem Chemother. 2000 Jul;11(4):291-301. doi: 10.1177/095632020001100405. Antivir Chem Chemother. 2000. PMID: 10950391
-
Resistance profile of the new nucleoside reverse transcriptase inhibitor apricitabine.J Antimicrob Chemother. 2010 Feb;65(2):213-7. doi: 10.1093/jac/dkp422. Epub 2009 Dec 9. J Antimicrob Chemother. 2010. PMID: 20007333 Review.
Cited by
-
Antiretroviral drugs: critical issues and recent advances.Indian J Pharmacol. 2012 May;44(3):288-98. doi: 10.4103/0253-7613.96296. Indian J Pharmacol. 2012. PMID: 22701234 Free PMC article.
-
Elucidating molecular interactions of L-nucleotides with HIV-1 reverse transcriptase and mechanism of M184V-caused drug resistance.Commun Biol. 2019 Dec 13;2:469. doi: 10.1038/s42003-019-0706-x. eCollection 2019. Commun Biol. 2019. PMID: 31872074 Free PMC article.
-
Status Presens of Antiviral Drugs And Strategies: Part I: DNA Viruses and Retroviruses.Adv Antivir Drug Des. 2007;5:1-58. doi: 10.1016/S1075-8593(06)05001-5. Epub 2007 Sep 2. Adv Antivir Drug Des. 2007. PMID: 32288472 Free PMC article. Review.
-
Nucleoside Analog Reverse-Transcriptase Inhibitors in Membrane Environment: Molecular Dynamics Simulations.Molecules. 2023 Aug 27;28(17):6273. doi: 10.3390/molecules28176273. Molecules. 2023. PMID: 37687102 Free PMC article.
-
Novel antiretroviral agents in HIV therapy.Curr Infect Dis Rep. 2006 Nov;8(6):489-96. doi: 10.1007/s11908-006-0024-6. Curr Infect Dis Rep. 2006. PMID: 17064643
References
-
- Anderson, P. L., T. N. Kakuda, and K. A. Lichtenstein. 2004. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin. Infect. Dis. 38:743-753. - PubMed
-
- Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose effect relationships: the combined effect of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. - PubMed
-
- Dalakas, M. C., C. Semino-Mora, and M. Leon-Monzon. 2001. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′,3′-dideoxycytidine (ddC). Lab. Investig. 81:1537-1544. - PubMed
-
- De Mendoza, C., O. Gallego, and V. Soriano. 2002. Mechanisms of resistance to antiretroviral drugs: clinical implications. AIDS Rev. 4:64-82. - PubMed
-
- de Muys, J.-M., H. Gourdeau, N. Nguyen-Ba, D. L. Taylor, P. S. Ahmed, T. Mansour, C. Locas, N. Richard, M. Wainberg, and R. F. Rando. 1999. Anti-human immunodeficiency virus type 1 activity, intracellular metabolism and pharmacokinetics evaluation of 2′-deoxy-3′-oxa-4′-thiocytidine. Antimicrob. Agents Chemother. 43:1835-1844. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

