Growth factor pleiotropy is controlled by a receptor Tyr/Ser motif that acts as a binary switch
- PMID: 16437163
- PMCID: PMC1383532
- DOI: 10.1038/sj.emboj.7600948
Growth factor pleiotropy is controlled by a receptor Tyr/Ser motif that acts as a binary switch
Abstract
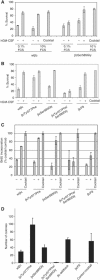
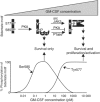
Pleiotropism is a hallmark of cytokines and growth factors; yet, the underlying mechanisms are not clearly understood. We have identified a motif in the granulocyte macrophage-colony-stimulating factor receptor composed of a tyrosine and a serine residue that functions as a binary switch for the independent regulation of multiple biological activities. Signalling occurs either through Ser585 at lower cytokine concentrations, leading to cell survival only, or through Tyr577 at higher cytokine concentrations, leading to cell survival as well as proliferation, differentiation or functional activation. The phosphorylation of Ser585 and Tyr577 is mutually exclusive and occurs via a unidirectional mechanism that involves protein kinase A and tyrosine kinases, respectively, and is deregulated in at least some leukemias. We have identified similar Tyr/Ser motifs in other cell surface receptors, suggesting that such signalling switches may play important roles in generating specificity and pleiotropy in other biological systems.
Figures




References
-
- Blume-Jensen P, Jiang G, Hyman R, Lee KF, O'Gorman S, Hunter T (2000) Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat Genet 24: 157–162 - PubMed
-
- Bulavin DV, Higashimoto Y, Demidenko ZN, Meek S, Graves P, Phillips C, Zhao H, Moody SA, Appella E, Piwnica-Worms H, Fornace AJ Jr (2003) Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat Cell Biol 5: 545–551 - PubMed
-
- Craparo A, Freund R, Gustafson TA (1997) 14-3-3 (ɛ) interacts with the insulin-like growth factor I receptor and insulin receptor substrate I in a phosphoserine-dependent manner. J Biol Chem 272: 11663–11669 - PubMed
-
- DeNichilo MO, Stewart AG, Vadas MA, Lopez AF (1991) Granulocyte-macrophage colony-stimulating factor is a stimulant of platelet-activating factor and superoxide anion generation by human neutrophils. J Biol Chem 266: 4896–4902 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

