Oral xanthines as maintenance treatment for asthma in children
- PMID: 16437447
- PMCID: PMC6999802
- DOI: 10.1002/14651858.CD002885.pub2
Oral xanthines as maintenance treatment for asthma in children
Abstract
Background: Xanthines have been used in the treatment of asthma as a bronchodilator, though they may also have anti-inflammatory effects. The current role of xanthines in the long-term treatment of childhood asthma needs to be reassessed.
Objectives: To determine the efficacy of xanthines (e.g. theophylline) in the maintenance treatment of paediatric asthma.
Search strategy: A search of the Cochrane Airways Group Specialised Register was undertaken with predefined search terms. Searches are current to May 2005.
Selection criteria: Randomised controlled trials,lasting at least four weeks comparing a xanthine with placebo, regular short-acting beta-agonist (SABA), inhaled corticosteroids (ICS), cromoglycate (SCG), ketotifen (KET) or leukotriene antagonist, in children with diagnosed with chronic asthma between 18 months and 18 years old.
Data collection and analysis: Two reviewers independently selected each study for inclusion in the review and extracted data. Primary outcome was percentage of symptom-free days.
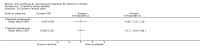
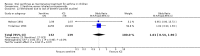
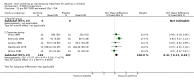
Main results: Thirty-four studies (2734 participants) of adequate quality were included. Xanthine versus placebo (17 studies): The proportion of symptom free days was larger with xanthine compared with placebo (7.97% [95% CI 3.41, 12.53]). Rescue medication usage was lower with xanthine, with no significant difference in symptom scores or hospitalisations. FEV1 , and PEF were better with xanthine. Xanthine was associated with non - specific side-effects. Data from behavioural scores were inconclusive. Xanthine versus ICS (four studies) : Exacerbations were less frequent with ICS, but no significant difference on lung function was observed. Individual studies reported significant improvements in symptom measures in favour of steroids, and one study reported a difference in growth rate in favour of xanthine. No difference was observed for study withdrawal or tremor. Xanthine was associated with more frequent headache and nausea. Xanthine versus regular SABA (10 studies): No significant difference in symptoms, rescue medication usage and spirometry. Individual studies reported improvement in PEF with beta-agonist. Beta-agonist treatment led to fewer hospitalisations and headaches. Xanthine was associated with less tremor. Xanthine versus SCG (six studies ): No significant difference in symptoms, exacerbations and rescue medication. Sodium cromoglycate was associated with fewer gastro-intestinal side-effects than xanthine. Xanthine versus KET (one study): No statistical tests of significance between xanthine and ketotifen were reported. Xanthine + ICS versus placebo + same dose ICS (three studies) : Results were conflicting due to clinical/methodological differences, and could not be aggregated.
Authors' conclusions: Xanthines as first-line preventer alleviate symptoms and reduce requirement for rescue medication in children with mild to moderate asthma. When compared with ICS they were less effective in preventing exacerbations. Xanthines had similar efficacy as single preventative agent compared with regular SABA and SCG. Evidence on AEs (adverse effects) was equivocal: there was evidence for increased AEs overall, but no evidence that any specific AE (including effects on behaviour and attention) occurred more frequently than with placebo. There is insufficient evidence from available studies to make firm conclusions about the effectiveness of xanthines as add-on preventative treatment to ICS, and there are no published paediatric studies comparing xanthines with alternatives in this role. Our data suggest that xanthines are only suitable as first-line preventative asthma therapy in children when ICS are not available. They may have a role as add-on therapy in more severe asthma not controlled by ICS, but further studies are needed to examine this, and to define the risk-benefit ratio compared with other agents.
Conflict of interest statement
None known.
Figures
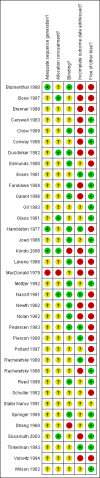










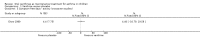

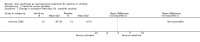










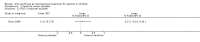





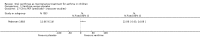
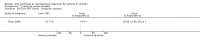



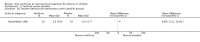


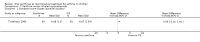
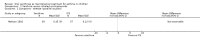
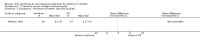
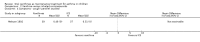
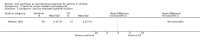
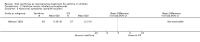
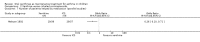


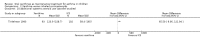



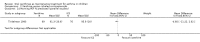
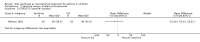
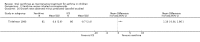









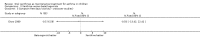











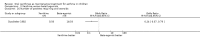



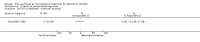
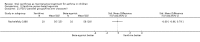







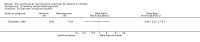
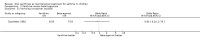




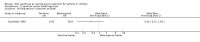

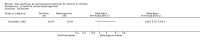










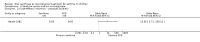
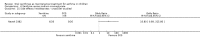



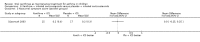










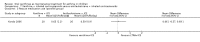

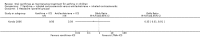
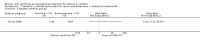


















Update of
References
References to studies included in this review
Blumenthal 1980 {published data only}
-
- Blumenthal I. A comparative trial of slow‐release aminophylline, salbutamol and a half dose combination in the prevention of childhood asthma. Journal of International Medical Research 1980;8(6):400‐3. - PubMed
Bose 1987 {published data only}
-
- Bose B, Cater JI, Clark RA. A once daily theophylline preparation in prevention of nocturnal symptoms in childhood asthma. European Journal of Pediatrics 1987;146(5):524‐7. - PubMed
Brenner 1988 {published data only}
-
- Brenner M, Berkowitz R, Marshall N, Strunk RC. Need for theophylline in severe steroid‐requiring asthmatics. Clinical Allergy 1988;18(2):143‐50. - PubMed
Carswell 1983 {published data only}
-
- Carswell F, Stratton D, Hughes AO, Fysh WJ, Robinson P. A controlled comparison of slow release theophylline, ketotifen and placebo in the prophylaxis of asthma in young children. Agents and Actions. Supplements 1983;13:141‐4. - PubMed
Chow 1989 {published data only}
-
- Chow OK, Fung KP. Slow‐release terbutaline and theophylline for the long‐term therapy of children with asthma: a Latin square and factorial study of drug effects and interactions. Pediatrics 1989;84(1):119‐25. - PubMed
Conway 1986 {published data only}
Dusdieker 1982 {published data only}
-
- Dusdieker L, Green M, Smith GD, Ekwo EE, Weinberger M. Comparison of orally administered metaproterenol and theophylline in the control of chronic asthma. Journal of Pediatrics 1982;101(2):281‐7. - PubMed
Edmunds 1980 {published data only}
Evans 1981 {published data only}
Furukawa 1984 {published data only}
-
- Furukawa CT, Shapiro GG, Bierman CW, Kraemer MJ, Ward DJ, Pierson WE. A double‐blind study comparing the effectiveness of cromolyn sodium and sustained‐release theophylline in childhood asthma. Pediatrics 1984;74(4):453‐9. - PubMed
Galant 1996 {published data only}
-
- Galant SP, Lawrence M, Meltzer EO, Tomasko M, Baker KA, Kellerman DJ. Fluticasone propionate compared with theophylline for mild‐to‐moderate asthma. Annals of Allergy, Asthma, & Immunology 1996;77(2):112‐8. - PubMed
Gil 1993 {published data only}
-
- Gil CA, Silveira MLM, Passos Soares FJ, Solé D, Naspitz C. Study of the effects of treatment with theophylline on the cognitive process and behaviour of children with bronchial asthma. Allergologia et Immunopathologia 1993;21(5):204‐6. - PubMed
Glass 1981 {published data only}
Hambleton 1977 {published data only}
-
- Hambleton G, Weinberger M, Taylor J, Cavanaugh M, Ginchansky E, Godfrey S, et al. Comparison of cromoglycate (cromolyn) and theophylline in controlling symptoms of chronic asthma. A collaborative study. Lancet 1977;1(8008):381‐5. - PubMed
Joad 1986 {published data only}
-
- Joad JP, Ahrens RC, Lindgren SD, Weinberger MM. Extrapulmonary effects of maintenance therapy with theophylline and inhaled albuterol in patients with chronic asthma. Journal of Allergy & Clinical Immunology 1986;78(6):1147‐53. - PubMed
-
- Joad JP, Ahrens RC, Lindgren SD, Weinberger MM. Relative efficacy of maintenance therapy with theophylline, inhaled albuterol, and the combination for chronic asthma. Journal of Allergy & Clinical Immunology 1987;79(1):78‐85. - PubMed
Kondo 2006 {published data only}
-
- Kondo N, Katsunuma T, Odajima Y, Morikawa A. A randomized open‐label comparative study of montelukast versus theophylline added to inhaled corticosteroid in asthmatic children. Allergology International 2006;55(3):287‐93. - PubMed
Levene 1986 {published data only}
-
- Levene S, McKenzie S. Once daily theophylline in childhood asthma. British Journal of Diseases of the Chest 1986;80(1):66‐71. - PubMed
MacDonald 1979 {published data only}
-
- MacDonald TH, McWilliam R. Monitoring response to bronchodilator therapy in asthma in childhood. Journal of International Medical Research 1979;7(Suppl 1):87‐92. - PubMed
Meltzer 1992 {published data only}
-
- Meltzer EO, Orgel A, Ellis EF, Eigen HN, Hemstreet MPB. Long‐term comparison of three combinations of albuterol, theophylline, and beclomethasone in children with chronic asthma. Journal of Allergy and Clinicial Immunology 1992;90(1):2‐11. - PubMed
Nassif 1981 {published data only}
-
- Nassif EG, Weinberger M, Thompson R, Huntley W. The value of maintenance theophylline in steroid‐dependent asthma. New England Journal of Medicine 1981;304(2):71‐5. - PubMed
Newth 1982 {published data only}
-
- Newth CJ, Newth CV, Turner JA. Comparison of nebulised sodium cromoglycate and oral theophylline in controlling symptoms of chronic asthma in pre‐school children: a double blind study. Australia and New Zealand Medical Journal 1982;12(3):232‐8. - PubMed
Nolan 1982 {published data only}
-
- Nolan G, Mindorff C, Reilly PA, Levison H. Comparison of the long‐term effect of fenoterol hydrobromide and theophylline syrups in pre‐school asthmatic children. Annals of Allergy 1982;49:93‐6. - PubMed
Pedersen 1983 {published data only}
-
- Pedersen S, Nathan E. Long‐term treatment of children with sustained‐release theophylline. European Respiratory Journal 1983;64(8):564‐70. - PubMed
Pierson 1990 {published data only}
-
- Pierson WE, LaForce CF, Bell TD, MacCosbe PE, Sykes RS, Tinkelman D. Long‐term, double‐blind comparison of controlled‐release albuterol versus sustained‐release theophylline in adolescents and adults with asthma. Journal of Allergy & Clinical Immunology 1990;85(3):618‐26. - PubMed
Pollard 1997 {published data only}
-
- Pollard SJ, Spector SL, Yancey SW, Cox FM, Emmett A. Salmeterol versus theophylline in the treatment of asthma. Annals of Allergy Asthma & Immunology 1997;78(5):457‐64. - PubMed
Rachelefsky 1980 {published data only}
-
- Rachelefsky GS, Katz RM, Mickey R, Siegel SC. Metaproterenol and theophylline in asthmatic children. Annals of Allergy 1980;45:207‐12. - PubMed
Rachelefsky 1986 {published data only}
-
- Rachelefsky GS, Wo J, Adelson J, Mickey MR, Spector SL, Katz RM, et al. Behavior abnormalities and poor school performance due to oral theophylline use. Pediatrics 1986;78(6):1133‐8. - PubMed
Reed 1998 {published data only}
-
- Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild‐to‐moderate asthma. Journal of Allergy and Clinical Immunology 1998;101(1 Pt 1):14‐23. - PubMed
Schuller 1982 {published data only}
-
- Schuller DE, Oppenheimer PJ. A comparison of metaproterenol and theophylline for control of childhood asthma. Clinical Pediatrics 1982;21(3):135‐42. - PubMed
Slater Nancy 1991 {published data only}
-
- Slater NF, Green M, Eigen H, Hui S, Taylor HG. Effects of theophylline on behaviour with children with chronic asthma. Pediatric Research 1991;29(Suppl):13A.
Springer 1985 {published data only}
-
- Springer C, Goldenberg B, Ben Dov I, Godfrey S. Clinical, physiologic, and psychologic comparison of treatment by cromolyn or theophylline in childhood asthma. Journal of Allergy & Clinical Immunology 1985;76(1):64‐9. - PubMed
Strang 1960 {published data only}
-
- Strang LB, Knox EG. Choline theophyllinate in children with asthma: a controlled trial. Lancet 1960;1:260‐2. - PubMed
Süssmuth 2003 {published data only}
-
- Süssmuth S, Freihorst J, Gappa M. Low‐dose theophylline in childhood asthma: a placebo‐controlled, double‐blind study. Pediatric Allergy & Immunology 2003;14(5):394‐400. - PubMed
-
- Süssmuth S, Gappa M, Freihorst J, Hardt H. Low‐dose theophylline in moderate pediatric asthma: a double‐blind placebo‐controlled study. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A542.
Tinkelman 1993 {published data only}
-
- Bender BG, Iklé DN, DuHamel T, Tinkelman D. Neuropsychological and behavioural changes in asthmatic children treated with beclomethasone dipropionate versus theophylline. Pediatrics 1998;101(3):355‐60. - PubMed
-
- Furukawa CC. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics 1998;102(1 Pt 2):265. - PubMed
-
- Tinkelman DG. Theophylline therapy for children with asthma. European Respiratory Review 1996;6(34):79‐83.
-
- Tinkelman DG, Reed CE, Nelson HS, Offord KP. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics 1993;92:64‐77. - PubMed
Volovitz 1994 {published data only}
-
- Volovitz B, Amir J, Malik H, Lerman M, Varsano I. Administration of half‐dose theophylline together with ketotifen to asthmatic children‐‐a double‐blind, placebo‐controlled study. Journal of Asthma 1994;31(1):27‐34. - PubMed
References to studies excluded from this review
Alvarez Sintes 1995 {published data only}
-
- Alvarez Sintes R, Alvarez Sintes R, Alvarez Castro MR. Sympathomimetics and theophylline: combined therapy [Simpaticomiméticos y teofilinas: ¿uso combinado?]. Anales de Medicina Interna 1995;12(9):438‐41. - PubMed
Avital 1991 {published data only}
-
- Avital A, Steljes DG, Pasterkamp H, Kryger M, Sanchez I, Cherniak V. Sleep quality in children with asthma treated with theophylline or cromolyn sodium. Pediatric Pharmacology and Therapeutics 1991;9(25):979‐84. - PubMed
Badiei 1975 {published data only}
-
- Badiei B, Faciane J, Sly M. Effect of theophylline ephedrine and their combination upon exercise‐induced airways obstruction. Annals of Allergy 1975;35:32‐6. - PubMed
Bellia 1988 {published data only}
Bender 1991 {published data only}
-
- Bender BG, Lerner JA, Ikle D, Comer C, Szefler S. Psychological change associated with theophylline treatment of asthmatic children: a 6 month study. Pediatric Pulmonology 1991;11:233‐42. - PubMed
Bender 1992 {published data only}
-
- Bender B, Milgrom H. Theophylline‐induced behavior change in children. Journal of the American Medical Association 1992;267(19):2621‐4. - PubMed
Bierman 1975 {published data only}
-
- Bierman CW, Pierson WE, Shapiro G. Exercise induced asthma ‐ pharmacological assessment of single drugs and drug combinations. Journal of Allergy & Clinial Immunology 1975;234(3):295‐8. - PubMed
Bierquist 1983 {published data only}
-
- Berquist WE, Rachelfsky GS, Rowshan N, Siegel S, Katz R, Welch M. Quantative gastrooesophageal reflux and pulmonary function in asthmatic children and normal adults receiving placebo, theophylline and metaproteranol sulfate. Journal of Allergy and Clinical Immunology 1984;73(2):253‐8. - PubMed
Boner 1984 {published data only}
-
- Boner AL, Zamo CR, Marchiori MM, Biancotto R, Antolini I, Vallone G. Comparison of nebulized ipratropium, salbutamol and cromoglycate solutions, cromoglycate inhaled powder, theophylline elixir and placebo in exercise induced asthma in children [Confronto fra nebulizzaaione di ipratropio, salbutamolo e cromoglicato in soluzione, cromoglicato in polvere inalabile, teofillina elisir e placebo nell'asma da esercizio dei bambini]. Giornale Italiano Malattie del Torace 1984;38(6):395‐9.
Brune 1991 {published data only}
-
- Brune J, Desfougères JL. Controlled release salbutamol, a new beta2 agonist. Results of a comparative study versus theophylline [Salbutamol à libération prolongée, un nouveau bêta 2 mimétique à action prolongée. Résultats d'une étude comparative verssu théophylline L.A.]. Allergie et Immunologie 1991;23(8):358‐64. - PubMed
Bundgaard 1982 {published data only}
-
- Bundgaard A, Weeke B. Long‐term treatment with oral sustained‐release theophylline. Allergy 1982;37:149‐54. - PubMed
Bundgaard 1990 {published data only}
-
- Bundgaard A, Schmidt A. A comparison of oral slow release theophylline and inhaled budesonide in adult asthmatics. Atemwegs‐ Und Lungenkrankheiten 1990;16(Suppl 1):S36‐S41.
Chapman 1989 {published data only}
-
- Chapman KR, Bryant D, Marlin GE, Mitchell C, Ruffin R, Inouye T, et al. A placebo‐controlled dose‐response study of enpofylline in the maintenance therapy of asthma. American Review of Respatory Disease 1989;139:688‐93. - PubMed
Crimi 1987 {published data only}
-
- Crimi N, Palermo F, Distefano SM, Vancheri C, Ciccallero C, Palermo B, et al. Relationship of serum theophylline concentrations to histamine‐induced bronchospasm. Respiration 1987;52:189‐94. - PubMed
Crimi 1995 {published data only}
-
- Crimi E, Orefice U, Benedetto F, Grassi V, Bruscasco V. Nedocromil sodium versus theophylline in the treatment of reversible obstructive airway disease. Annals of Allergy, Asthma and Immunology 1995;74:501‐8. - PubMed
Darke 1970 {published data only}
-
- Darke CS, Picton EA. A delayed‐release theophylline‐noscapine formulation for the relief of airways obstruction. Practitioner 1970;204(220):276‐81. - PubMed
Edwards 1995 {published data only}
-
- Edwards TB, Dockhorn RJ, Wagner DE, Fiddes RA, Grossman J, Menendez R, et al. Efficacy of once daily extended‐release theophylline in decreasing the use of inhaled ß2 agonists in stable, mild‐to‐moderate asthma patients. Annals of Allergy, Asthma & Immunology. 1995;75:409‐16. - PubMed
Elias‐Jones 1984 {published data only}
Eriksson 1983 {published data only}
-
- Eriksson NE, Haglind K, Ewald U. Combined theophylline/beta‐agonists maintenance therapy in chronic asthma. European Journal of Respiratory Disease 1983;64(3):172‐7. - PubMed
Evans 1997 {published data only}
-
- Crain E, Evans DJ, et al. A comparison of low‐dose inhaled budesonide plus theophylline and high‐dose inhaled budesonide for moderate asthma. Emergency and Office Pediatrics 1998;11(2):77‐8. - PubMed
-
- Evans DJ, Taylor DA, Zetterstrom O, Chung KF, O'Connor BJ, Barnes PJ. A comparison of low‐dose inhaled budesonide plus theophylline and high‐dose inhaled budesonide for moderate asthma. New England Journal of Medicine 1997;337(20):1412‐8. - PubMed
Fabbri 1996 {published data only}
-
- Fabbri LM, Piatella M, Caramoni G, Ciaccia A. Oral versus inhaled asthma therapy. Drugs 1996;52(Suppl 6):20‐8. - PubMed
Furukawa 1988 {published data only}
-
- Furukawa CT. Comparative trials including a beta2 adrenergic agonist, a methylxanthine, and a mast cell stabilizer. Annals of Allergy 1988;60:472‐6. - PubMed
Furukawa 1988a {published data only}
-
- Furukawa CT, DuHamel TR, Weimer L, Shapiro GG, Pierson WE, Bierman W. Cognitive and behavioural findings in children taking theophylline. Journal of Allergy & Clinical Immunology 1988;60:83‐8. - PubMed
Godley 1991 {published data only}
-
- Godley PJ, Karboski JA, Godley SE, Edwards GA, Moore ES, Sagraves R. Evaluation of three theophylline dosing methods in pediatric patients. DICP 1991;25(2):179‐85. - PubMed
Goldthorpe 1964 {published data only}
-
- Goldthorpe AM, Milton RJ, Moffat RJ, Peatfield BJ, Ryde DH. Clinical trial of a new theophylline preparation. Practitioner 1964;193:789‐92. - PubMed
Groggins 1980 {published data only}
Guo 2002 {published data only}
-
- Guo JG, Cheng ST. The efficacy of low‐dose oral aminophylline combined with inhaled corticosteroid in the treatment of asthmatic children in remission period. Acta Academic Medicine Xuzhou 2002;22(4):349‐51.
Haahtela 1998 {published data only}
-
- Haahtela T. The long‐term influence of therapeutic interventions in asthma with emphasis on inhaled steroids and early disease. Clinical and Experimental Allergy 1998;28(Suppl 5):133‐40. - PubMed
Heimlich 1964 {published data only}
-
- Heimlich EM, Siegel SC. Clinical and laboratory evaluation of an antiasthmatic preparation with prolonged action. Journal of Allergy & Clinical Immunology 1964;35:27‐37. - PubMed
Hendeles 1995 {published data only}
-
- Hendeles L, Harman E, Huang D, O'Brien R, Blake K, Delafuente J. Theophylline attenuation of airway responses to allergen: comparison with cromolyn metered‐dose inhaler. Journal of Allergy & Clinical Immunology 1995;95(2):505‐14. - PubMed
Hoffmann‐Streb 1993 {published data only}
-
- Hoffmann‐Streb A, Niggemann B, Wahn U. Investigations in to the protective effect of theophylline in paediatric exercise‐induced bronchoconstriction [Untersuchingen zum protektiven Effekt von Theophyllin bei Anstrengungsasthma im Kindesalter]. Klinische Pädiatrie 1993;205:99‐102. - PubMed
Ibáñez 1994 {published data only}
-
- Ibáñez MD, Laso MT, Alonso E, Muñoz MC, Sastre J. Effect of theophylline on airway responsiveness to methacholine and on exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 1994;73:357‐63. - PubMed
Irvin 2007 {published data only}
-
- Irvin CG, Kaminsky DA, Anthonisen NR, Castro M, Hanania NA, Holbrook JT, et al. Clinical trial of low‐dose theophylline and montelukast in patients with poorly controlled asthma. American Journal of Respiratory & Critical Care Medicine 2007;175(3):235‐42. - PubMed
Jain 1993 {published data only}
-
- Jain NK, Sharma MD, Garg VK, Sharma TN, Devpura K. Is combined therapy of sympathomimetics and theophylline indicated?. Journal of Asthma 1993;30(1):29‐35. - PubMed
Jatulis 1998 {published data only}
-
- Jatulis DE, Meng YY, Elashoff RM, Schocket AL, Evans RM, Hasan AG, et al. Preventive pharmacologic therapy among asthmatics: five years after publication of guidelines. Annals of Allergy Asthma & Immunology 1998;81:82‐8. - PubMed
Johnson 1998 {published data only}
-
- Johnson FN, Barnes NC. Fluticasone propionate in the treatment of asthma in adults and adolescents. Review of Contemporary Pharmacotherapy 1998;9:551‐67.
Jonkman 1984 {published data only}
-
- Jonkman JH, Boon WJV, Schoenmaker R, et al. The absolute bioavailability of a new pediatric sustained release theophylline tablet, when given as whole or divided tablets. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1984;22(9):506‐10. - PubMed
Katz 1978 {published data only}
-
- Katz RM, Rachelfsky GS, Siegel S. The effectiveness of the short‐ and long‐term use of crystallized theophylline in asthmatic children. The Journal of Pediatrics 1978;92(4):663‐7. - PubMed
Koyande 1993 {published data only}
-
- Koyande DN, Shah SP, Hingorani M, Ailani RK, Haran A, Kadge KM. Comparative efficacy of oral sustained release bronchodilator in stable asthmatics. Indian Journal of Chest Disease and Allied Sciences 1993;35(2):51‐7. - PubMed
Kreisman 1984 {published data only}
-
- Kreisman H, Cohen C, Ghezzo H, Vickerson F, Frank H, Wolklove N. Combined therapy with ipratropium and theophylline in asthma. Annals of Allergy 1984;52:90‐3. - PubMed
Laursen 1985 {published data only}
-
- Laursen LC, Taudorf E, Gnosspelius Y, Gymose E, Weeke B. Long‐term oral therapy of asthma with terbutaline and theophylline, alone and combined. European Journal of Respiratory Disease 1985;66:82‐90. - PubMed
Lönnerholm 1981 {published data only}
Marín 1990 {published data only}
-
- Marin JM, Carrizo S, Garcia R, Ejea MV. A second drug in non‐atopic asthma insufficiently controlled with beta 2‐adrenergic stimulants: budesonide versus theophylline. Medicina Clinica 1990;95(18):684‐8. - PubMed
Muir 1992 {published data only}
-
- Muir JF, Bertin L, Georges D, et al. Salmeterol versus slow‐release theophylline combined with ketotifen in nocturnal asthma: a multicentre trial. European Respiratory Journal 1992;5:1197‐200. - PubMed
-
- Muir JF, Georges D, et al. The effect of salmeterol in nocturnal asthma: a comparative study with a combination of theophylline and ketotifen. Revue des Maladies Respiratoires 1992;9:R23‐6. - PubMed
Nicholson 1979 {published data only}
-
- Nicholson EM, Laszlo G. Subjective and objective changes noted by patients with bronchial asthma taking slow‐release aminophylline. Journal of International Medical Research 1979;7(Suppl 1):32‐3. - PubMed
Paggiaro 1996 {published data only}
-
- Paggiaro PL, Giannini D, Franco A, Testi R, et al. Comparison of inhaled salmeterol and individually dose‐titrated slow‐release theophylline in patients with reversible airway obstruction. European Respiratory Journal 1996;9:1689‐95. - PubMed
Pastorello 1998 {published data only}
-
- Pastorello EA, Mauro M, Incorvaia C. Comparison of efficacy and safety of inhaled salmeterol and slow‐release oral theophylline in patients with moderate/severe asthma [Confronto di efficacia e tollerabilità tra salmeterolo per via inalatoria e teofillina orale a lento rilascio in pazienti affetti da asma grado medio/grave]. L'Internista 1998;6:101‐7.
Pedersen 1985 {published data only}
-
- Pedersen S. Treatment of nocturnal asthma in children with a single dose of sustained‐release theophylline taken after supper. Clinical Allergy 1985;15:79‐85. - PubMed
Pednekar 1998 {published data only}
-
- Pednekar SJ, Drago SD, Sapre AS, Nabar ST, Pai‐dhungat AJ, Iyengar V, et al. Bronchial asthma: a comparison of pulmonary functions and biochemical parameters following inhaled salmeterol versus slow release oral theophylline. The Indian Practitioner 1998;51:182‐6.
Pereira 1988 {published data only}
-
- Castro Pereira CA, dos Santos Friere JA, Rassi RH, Pereira LF. Is treatment with oral steroids necessary in the treatment of acute asthma? [Sao os corticosteròides necessários no tratamento da asma aguda nao grave?]. Revista Paulista de Medicina 1988;106(1):28‐34. - PubMed
Pijaskic‐Kamenov 2001 {unpublished data only}
-
- Pijaskic‐Kamenov SS, Filipovic MD, Kamenov BA, Cekic SS. Sustained release theophylline added to fluticasone propionate in the treatment of paediatric asthma. European Respiratory Journal 2001;18(Suppl 33):123s.
Rachelfsky 1978 {published data only}
-
- Rachelfsky GS, Katz RM, Siegel SC. A sustained release theophylline preparation: efficacy in childhood asthma with low serum theophylline levels. Annals of Allergy 1978;40:252‐7. - PubMed
Rappaport 1989 {published data only}
-
- Rappaport L, Coffman H, Guare R, Fenton R, DeGraw C, Twarog F. Effects of theophylline on behaviour and learning in children with asthma. American Journal of Disease in Childhood 1989;143:368‐72. - PubMed
Roberts 1986 {published data only}
-
- Roberts JR, Desai HI, Gillespie CA, Simons ER. Sustained‐release terbutaline versus sustained‐release theophylline in young patients with asthma. American Journal of Disease in Childhood 1986;140:650‐3. - PubMed
Roberts 2003 {published data only}
Roddick 1979 {published data only}
-
- Roddick LG, South RT, Mellis CM. Value of combining an oral sympathomimetic agent with oral theophylline in asthmatic children. Medical Journal of Australia 1979;2:118, 153‐4.
Schlieper 1991 {published data only}
-
- Schlieper A, Alcock D, Beaudry P, Feldman W, Lelkin L. Effect of therapeutic plasma concentrations of theophylline on behaviour, cognitive processing, and affect in children with asthma. Pediatric Pharmacology and Therapeutics 1991;118:449‐55. - PubMed
Schnabel 1989 {published data only}
-
- Schnabel D, Sybrecht G. Treatment of nocturnal asthma [Therapie des nächtlichen Asthma bronchiale]. Pneumologie 1989;43:635‐8. - PubMed
Shaffer 1997 {published data only}
-
- Shaffer DN, Mansmann PT. Leukotriene inhibtion and advances in the treatment of asthma: a pharamcological review. Pediatric Asthma, Allergy and Immunology 1997;11(4):171‐9.
Sienra Monge 1994 {published data only}
-
- Sienra Monge JJL, Prieto Ursúa L, Gerardo Sol Monterrey E, Estela del Rio Navarro B, Paredes Novelo MC. Valoración de la eficacia y seguridad de la mepifilina en solución oral en ninos con crisis de asma leve a moderada. Allergologia et Immunopathologia 1994;22(1):3‐8. - PubMed
Stein 1993 {published data only}
-
- Stein MA, Lerner CA. Behavioral and cognitive effect of theophylline: a dose‐response study. Annals of Allergy 1993;70:135‐40. - PubMed
Sullivan 1994 {published data only}
-
- Costello J. Theophylline in the treatment of mild asthma. European Respiratory Review 1996;6(34):84‐7.
-
- Sullivan P, Bekir S, Jaffar Z, Page C, Jeffery P, Costello J. Anti‐inflammatory effects of low‐dose oral theophylline in atopic asthma. Lancet 1994;343:1006‐8. - PubMed
Trakultivakorn 1999 {published data only}
-
- Trakultivakorn M, Kanthawatana S, Tontayapiwat A, Jiraporncharoen K. Comparative study of the pharmacokinetic characteristics of slow release theophylline oral preparations in Thai children with persistent asthma. Asian Pacific Journal of Allergy & Immunology 1999;17(4):255‐9. - PubMed
Ukena 1998 {published data only}
-
- Ukena D, Harnest U, Sakalauskas R, Magyar P, Vetter N, Steffen H, et al. Additional effect of theophylline in treatment with inhaled steroids in comparison with double‐dose of inhaled steroid in asthma. Pneumologie 1998;52(7):377‐84. - PubMed
Van Asperen 1981 {published data only}
-
- Asperen PP, Mellis CM, South RT, Simpson SJ. Value of combining B2 sympathomimetic metered aerosol and oral theophylline in children with asthma. Medical Journal of Australia 1981;1(12):643‐4. - PubMed
Van Caillie 1988 {unpublished data only}
-
- Cailie BD, Melis K. Pharmacodynamic study of a long acting oral theophylline (Theodur) and a controlled release formulation of oral beta‐mimetic (volmax) versus placebo in asthmatic children. European Respiratory Journal 1988;1(Suppl 2):356s.
Vilkka 1990 {published data only}
-
- Vilkka V, Brander P, Hakulinen A, Laitinen J, Sahlström, Aalto E, Silvasti M, et al. Once‐daily theophylline in the treatment of nocturnal asthma. European Journal of Clinical Pharmacology 1990;39:241‐3. - PubMed
Ward 1993 {published data only}
-
- Costello J. Theophylline in the treatment of mild asthma. European Respiratory Review 1996;6(34):84‐7.
-
- Ward AJ, McKenniff M, Evans JM, Page CP, Costello JF. Theophylline ‐ an immunomodulatory role in asthma?. American Review of Respiratory Disease 1993;147:518‐23. - PubMed
Weinberger 1974 {published data only}
-
- Weinberger M, Bronsky EA. Evaluation of oral bronchodilator therapy in asthmatic children: bronchodilators in asthmatic children. Journal of Pediatrics 1974;84(3):421‐27. - PubMed
Wheatley 1982 {published data only}
-
- Wheatley D. Clenbuterol ('Spiropent') a long‐acting bronchodilator. Current Medical Research and Opinion 1982;8(2):113‐9. - PubMed
Youngchaiyud 1995 {published data only}
-
- Youngchaiyud P, Permpikul C, Suthamsmai T, Wong E. A double‐blind comparison of inhaled budesonide, long‐acting theophylline, and their combination in treatment of nocturnal asthma cells in patients suffering from allergic rhinitis. Allergy 1995;50:28‐33. - PubMed
Zeitlin 1988 {published data only}
-
- Zeitlin S, Foulkes D, Rolles C. Effect of salbutamol controlled release (Volmax(r); SCR) and theophylline SR tablets on the sleep EEG of asthmatic children. European Respiratory Journal 1988;1(Suppl 2):334s.
References to studies awaiting assessment
El Kateeb 1986 {published data only}
-
- Kateeb S. A comparative study of the use of regular and sustained release preparations of theophylline in children with bronchial asthma. Bulletin of Alexandria Faculty of Medicine 1986;22(4):1079‐89.
Additional references
Adams 2005
BTS 2003
-
- British Thoracic Society. British Guidelines on Asthma Management. Thorax 2003;58(Suppl 1):i1‐i94. - PubMed
Ducharme 2004
Greening 1994
-
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher‐dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet 1994;344(8917):219‐24. - PubMed
Handbook 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008.
Hermann 1937
-
- Hermann G, Aynesworth MB. Successful treatment of persistent extreme dyspnea "status asthmaticus": use of theophylline ethylene diamine (aminophylline, USP) intravenously. The Journal of Laboratory and Clinical Medicine 1937;23:135‐48. - PubMed
Higgins 2003
Ind 2003
-
- Ind PW, Dal Negro R, Colman NC, Fletcher CP, Browning D, James MH. Addition of salmeterol to fluticasone propionate treatment in moderate to severe asthma. Respiratory Medicine 2003;97:555‐62. - PubMed
IPACG 1992
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;134(17):1‐12. - PubMed
Kabra 2003
-
- Kabra SK, Lodha R. Long‐term management of asthma. Indian Journal of Pediatrics 2003;70(1):63‐72. - PubMed
NHLBI 2002
-
- National Heart Lung and Blood Institute. Global strategy for asthma management and prevention. NHLBI/WHO Workshop Report. www.nhlbi.org. 2002.
Ni Chroinin 2005
-
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma (Cochrane review). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] - DOI - PubMed
Pauwels 1989
-
- Pauwels RA. New aspects of the therapeutic potential of theophylline in asthma. Journal of Allergy & Clinical Immunology 1989;83:548‐53. - PubMed
Stein 1996
-
- Stein MA, Krasowski M, Leventhal BL, Phillips W, Bender BG. Behavioral and cognitive effects of methylxanthines. A meta‐analysis of theophylline and caffeine. Archives of Pediatrics & Adolescent Medicine 1996;150(3):284‐8. - PubMed
Vassallo 1998
-
- Vassallo R, Lipsky JJ. Theophylline: recent advances in the understanding of its mode of action and uses in clinical practice. Mayo Clinic Proceedings 1998;73:346‐54. - PubMed
Verberne 1998
-
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1998;158(1):213‐9. - PubMed
Walters 2007
-
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

