Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial
- PMID: 16439370
- PMCID: PMC1363908
- DOI: 10.1136/bmj.38733.466748.7C
Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial
Abstract
Objective: To determine the efficacy and safety of the anticoagulant fondaparinux in older acute medical inpatients at moderate to high risk of venous thromboembolism.
Design: Double blind randomised placebo controlled trial.
Setting: 35 centres in eight countries.
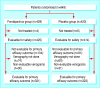
Participants: 849 medical patients aged 60 or more admitted to hospital for congestive heart failure, acute respiratory illness in the presence of chronic lung disease, or acute infectious or inflammatory disease and expected to remain in bed for at least four days.
Interventions: 2.5 mg fondaparinux or placebo subcutaneously once daily for six to 14 days.
Outcome measure: The primary efficacy outcome was venous thromboembolism detected by routine bilateral venography along with symptomatic venous thromboembolism up to day 15. Secondary outcomes were bleeding and death. Patients were followed up at one month.
Results: 425 patients in the fondaparinux group and 414 patients in the placebo group were evaluable for safety analysis (10 were not treated). 644 patients (75.9%) were available for the primary efficacy analysis. Venous thrombembolism was detected in 5.6% (18/321) of patients treated with fondaparinux and 10.5% (34/323) of patients given placebo, a relative risk reduction of 46.7% (95% confidence interval 7.7% to 69.3%). Symptomatic venous thromboembolism occurred in five patients in the placebo group and none in the fondaparinux group (P = 0.029). Major bleeding occurred in one patient (0.2%) in each group. At the end of follow-up, 14 patients in the fondaparinux group (3.3%) and 25 in the placebo group (6.0%) had died.
Conclusion: Fondaparinux is effective in the prevention of asymptomatic and symptomatic venous thromboembolic events in older acute medical patients. The frequency of major bleeding was similar for both fondaparinux and placebo treated patients.
Figures
Comment in
-
Can fondaparinux safely prevent the occurrence of venous thromboembolism in acutely ill medical patients?Nat Clin Pract Cardiovasc Med. 2006 Jul;3(7):358-9. doi: 10.1038/ncpcardio0582. Nat Clin Pract Cardiovasc Med. 2006. PMID: 16810167 No abstract available.
-
Thromboprophylaxis for adults in hospital: A mess for medical patients.BMJ. 2007 Jun 2;334(7604):1127. doi: 10.1136/bmj.39226.461516.3A. BMJ. 2007. PMID: 17540915 Free PMC article. No abstract available.
References
-
- Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT study. Arch Intern Med 1991;151: 933-8. - PubMed
-
- Kniffin WD Jr, Baron JA, Barrett J, Birkmeyer JD, Anderson FA Jr. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994;154: 861-6. - PubMed
-
- Weinmann EE, Salzman EW. Deep-vein thrombosis. N Engl J Med 1994;331: 1630-41. - PubMed
-
- Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002;162: 1245-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases