Generation of CD8+ T-cell responses by a recombinant nonpathogenic Mycobacterium smegmatis vaccine vector expressing human immunodeficiency virus type 1 Env
- PMID: 16439521
- PMCID: PMC1367151
- DOI: 10.1128/JVI.80.4.1645-1652.2006
Generation of CD8+ T-cell responses by a recombinant nonpathogenic Mycobacterium smegmatis vaccine vector expressing human immunodeficiency virus type 1 Env
Abstract
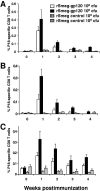
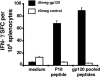
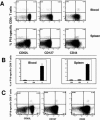
Because the vaccine vectors currently being evaluated in human populations all have significant limitations in their immunogenicity, novel vaccine strategies are needed for the elicitation of cell-mediated immunity. The nonpathogenic, rapidly growing mycobacterium Mycobacterium smegmatis was engineered as a vector expressing full-length human immunodeficiency virus type 1 (HIV-1) HXBc2 envelope protein. Immunization of mice with recombinant M. smegmatis led to the expansion of major histocompatibility complex class I-restricted HIV-1 epitope-specific CD8(+) T cells that were cytolytic and secreted gamma interferon. Effector and memory T lymphocytes were elicited, and repeated immunization generated a stable central memory pool of virus-specific cells. Importantly, preexisting immunity to Mycobacterium bovis BCG had only a marginal effect on the immunogenicity of recombinant M. smegmatis. This mycobacterium may therefore be a useful vaccine vector.
Figures



References
-
- Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. - PubMed
-
- Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351:479-482. - PubMed
-
- Andersson, G. E., and P. M. Sharp. 1996. Codon usage in the Mycobacterium tuberculosis complex. Microbiology 142:915-925. - PubMed
-
- Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. - PMC - PubMed
-
- Bange, F. C., F. M. Collins, and W. R. Jacobs, Jr. 1999. Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber. Lung Dis. 79:171-180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

