A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques
- PMID: 16439526
- PMCID: PMC1367169
- DOI: 10.1128/JVI.80.4.1688-1699.2006
A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques
Abstract
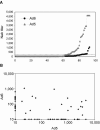
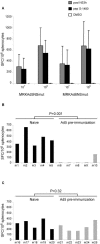
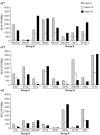
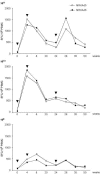
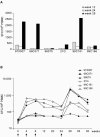
Success in resolving hepatitis C virus (HCV) infection has been correlated to vigorous, multispecific, and sustained CD8(+) T-cell response in humans and chimpanzees. The efficacy of inducing T-cell-mediated immunity by recombinant serotype 5 adenovirus vector has been proven in many animal models of infectious diseases, but its immunogenicity can be negatively influenced by preexisting immunity against the vector itself. To evaluate the less prevalent adenovirus serotype 6 (Ad6) as an alternative vector for and HCV vaccine development, we have generated serotype 5 and 6 adenoviral vectors directing expression of the nonstructural region of HCV (MRKAd5-NSmut and MRKAd6-NSmut). Immunogenicity studies in mice showed that the two vectors induced comparable T-cell responses but that only MRKAd6-NSmut was not suppressed in the presence of anti-Ad5 immunity. In contrast, preexisting anti-Ad5 immunity dramatically blunted the immunogenicity of the serotype 5-based HCV vector. Furthermore, MRKAd6-NSmut showed equivalent potency, breadth, and longevity of HCV-specific T-cell responses in rhesus macaques as the corresponding Ad5-based vector over a wide range of doses and was capable of boosting DNA-primed animals even if administered at low doses. These data support the use of the MRKAd6-NSmut for anti-HCV immunotherapy and, more generally, for the Ad6 serotype as a better genetic vaccine vehicle than Ad5.
Figures


References
-
- Arribillaga, L., A. L. de Cerio, P. Sarobe, N. Casares, M. Gorraiz, A. Vales, O. Bruna-Romero, F. Borras-Cuesta, G. Paranhos-Baccala, J. Prieto, J. Ruiz, and J. J. Lasarte. 2002. Vaccination with an adenoviral vector encoding hepatitis C virus (HCV) NS3 protein protects against infection with HCV-recombinant vaccinia virus. Vaccine 21:202-210. - PubMed
-
- Arribillaga, L., P. Sarobe, A. Arina, M. Gorraiz, F. Borras-Cuesta, J. Ruiz, J. Prieto, L. Chen, I. Melero, and J. J. Lasarte. 2005. Enhancement of CD4 and CD8 immunity by anti-CD137 (4-1BB) monoclonal antibodies during hepatitis C vaccination with recombinant adenovirus. Vaccine 23:3493-3499. - PubMed
-
- Aste-Amezaga, M., A. J. Bett, F. Wang, D. R. Casimiro, J. M. Antonello, D. K. Patel, E. C. Dell, L. L. Franlin, N. M. Dougherty, P. S. Bennett, H. C. Perry, M. E. Davies, J. W. Shiver, P. M. Keller, and M. D. Yeager. 2004. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum. Gene Ther. 15:293-304. - PubMed
-
- Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. - PubMed
-
- Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479-1487. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

