Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice
- PMID: 16439559
- PMCID: PMC1367143
- DOI: 10.1128/JVI.80.4.2034-2044.2006
Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice
Abstract
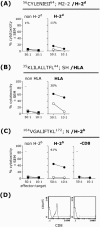
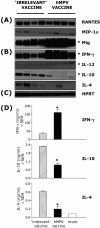
Human metapneumovirus (hMPV) has emerged as an important human respiratory pathogen causing upper and lower respiratory tract infections in young children and older adults. In addition, hMPV infection is associated with asthma exacerbation in young children. Recent epidemiological evidence indicates that hMPV may cocirculate with human respiratory syncytial virus (hRSV) and mediate clinical disease similar to that seen with hRSV. Therefore, a vaccine for hMPV is highly desirable. In the present study, we used predictive bioinformatics, peptide immunization, and functional T-cell assays to define hMPV cytotoxic T-lymphocyte (CTL) epitopes recognized by mouse T cells restricted through several major histocompatibility complex class I alleles, including HLA-A*0201. We demonstrate that peptide immunization with hMPV CTL epitopes reduces viral load and immunopathology in the lungs of hMPV-challenged mice and enhances the expression of Th1-type cytokines (gamma interferon and interleukin-12 [IL-12]) in lungs and regional lymph nodes. In addition, we show that levels of Th2-type cytokines (IL-10 and IL-4) are significantly lower in hMPV CTL epitope-vaccinated mice challenged with hMPV. These results demonstrate for the first time the efficacy of an hMPV CTL epitope vaccine in the control of hMPV infection in a murine model.
Figures



References
-
- Barr, J., P. Chambers, C. R. Pringle, and A. J. Easton. 1991. Sequence of the major nucleocapsid protein gene of pneumonia virus of mice: sequence comparisons suggest structural homology between nucleocapsid proteins ofpneumoviruses, paramyxoviruses, rhabdoviruses and filoviruses. J. Gen. Virol. 72:677-685. - PubMed
-
- Biacchesi, S., M. H. Skiadopoulos, G. Boivin, C. T. Hanson, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2003. Genetic diversity between human metapneumovirus subgroups. Virology 315:1-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

