1alpha,25-Dihydroxyvitamin D3 downmodulates the functional differentiation of Th1 cytokine-conditioned bone marrow-derived dendritic cells beneficial for cytotoxic T lymphocyte generation
- PMID: 16441425
- PMCID: PMC11158372
- DOI: 10.1111/j.1349-7006.2006.00144.x
1alpha,25-Dihydroxyvitamin D3 downmodulates the functional differentiation of Th1 cytokine-conditioned bone marrow-derived dendritic cells beneficial for cytotoxic T lymphocyte generation
Abstract
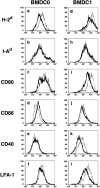
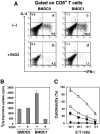
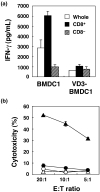
Various dendritic cell subsets are induced from bone marrow cells under different cytokine conditions. We have demonstrated previously that the Th1-cytokine-conditioned bone marrow-derived dendritic cell (BMDC) subset BMDC1 (generated in the presence of granulocyte-macrophage colony-stimulating factor [GM-CSF] + interleukin [IL]-3 + interferon [IFN]-gamma+ IL-12) induces a much stronger type 1 immune response than BMDC0 (GM-CSF + IL-3). In the present study, we investigated the effect of 1alpha,25-dihydroxyvitamine D3 (VitD3), which is a known immunomodulating drug, on the differentiation of BMDC subsets. The addition of VitD3 significantly influenced the functional differentiation of BMDC1 compared with BMDC0. Specifically, the addition of VitD3 greatly decreased the expression levels of MHC class I, CD80, CD40 and leukocyte function-associated antigen (LFA)-1 molecules on BMDC1. In addition, VitD3-treated BMDC1 (VD3-BMDC1) almost completely lost their immunostimulating activity for inducing type 1 immunity and cytotoxic T lymphocyte generation. A failure in the induction of type 1 immunity by VD3-BMDC1 appeared to be due to the following: (i) the expression of co-stimulatory molecules on VD3-BMDC1 was strongly downmodulated compared with BMDC1 generated without VitD3; and (ii) VD3-BMDC1 showed significantly lower mRNA expression of IFN-gamma and IFN-beta, factors that are essential for cytotoxic T lymphocyte induction. VitD3 inhibited the differentiation of functionally competent BMDC1 during the early phase of differentiation but not during the late differentiation period. A possible reason for the inhibition of BMDC1 differentiation by VitD3 is reduced phosphorylation of STAT1 during early differentiation. Taken together, VitD3 strongly suppressed T-cell responses by inhibiting functional differentiation of precursor dendritic cells into functional BMDC1 that are feasible for inducing Th1-dependent cellular immunity.
Figures



References
-
- Banchereau J, Steinman RB. Dendritic cells and the control of immunity. Nature 1998; 392: 245–52. - PubMed
-
- Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol 1999; 19: 12–25. - PubMed
-
- Sato M, Iwakabe K, Ohta A et al. Functional heterogeneity among bone marrow‐derived dendritic cells conditioned by Th1‐ and Th2‐biasing cytokines for the generation of allogeneic cytotoxic T lymphocytes. Int Immunol 2000; 12: 335–42. - PubMed
-
- Sato M, Chamoto K, Nishimura T. A novel tumor‐vaccine cell therapy using bone marrow‐derived dendritic cell type 1 and antigen‐specific Th1 cells. Int Immunol 2003; 15: 837–43. - PubMed
-
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL‐10‐treated dendritic cells. J Immunol 1997; 159: 4772–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

