rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion
- PMID: 16442847
- PMCID: PMC1712670
- DOI: 10.1016/j.ymthe.2005.12.002
rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion
Abstract
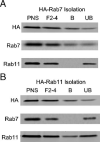
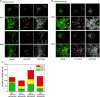
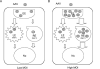
Inefficient trafficking of recombinant adeno-associated virus type-2 (rAAV2) to the nucleus is a major barrier for transduction. Using imaging and subcellular fractionation techniques, we evaluated the extent of rAAV2 movement through the late (Rab7) and recycling (Rab11) endosomes. Following rAAV2 infection of HeLa cells, immunoisolation of HA-Rab7- or HA-Rab11-tagged endosomes and intracellular colocalization of Cy3-labeled rAAV2 with EGFP-Rab7 or EGFP-Rab11 markers demonstrated dose-dependent trafficking of rAAV2 through the recycling and late endosomal compartments. At low multiplicities of infection (m.o.i. 100 genomes/cell), rAAV2 predominantly trafficked to the Rab7 compartment. In contrast, rAAV2 predominantly trafficked to the recycling endosome at 100-fold higher m.o.i. siRNA studies inhibiting either Rab7 or Rab11 demonstrated that reducing Rab11 protein levels more significantly inhibited rAAV2 transduction on a per genome basis compared to inhibition of Rab7. Dose-response curves, comparing the m.o.i. of AV2Luc infection to relative transduction, also supported the hypothesis that viral movement through the Rab11 compartment at high m.o.i. is more competent for transgene expression ( approximately 100-fold) than virus that moves through the Rab7 compartment at low m.o.i. These findings suggest that strategies to shunt viral movement from the late to the recycling endosome may be effective at increasing viral transduction for gene therapy.
Figures




Similar articles
-
Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation.J Virol. 2001 Feb;75(4):1824-33. doi: 10.1128/JVI.75.4.1824-1833.2001. J Virol. 2001. PMID: 11160681 Free PMC article.
-
Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors.J Cell Sci. 2004 Jul 1;117(Pt 15):3107-17. doi: 10.1242/jcs.01168. Epub 2004 Jun 9. J Cell Sci. 2004. PMID: 15190120
-
Rab11 and Lysotracker Markers Reveal Correlation between Endosomal Pathways and Transfection Efficiency of Surface-Functionalized Cationic Liposome-DNA Nanoparticles.J Phys Chem B. 2016 Jul 7;120(26):6439-53. doi: 10.1021/acs.jpcb.6b04441. Epub 2016 Jun 3. J Phys Chem B. 2016. PMID: 27203598 Free PMC article.
-
Recycling Endosomes and Viral Infection.Viruses. 2016 Mar 8;8(3):64. doi: 10.3390/v8030064. Viruses. 2016. PMID: 27005655 Free PMC article. Review.
-
Rab11-mediated recycling endosome role in nervous system development and neurodegenerative diseases.Int J Neurosci. 2021 Oct;131(10):1012-1018. doi: 10.1080/00207454.2020.1761354. Epub 2020 May 6. Int J Neurosci. 2021. PMID: 32329391 Review.
Cited by
-
Optimization of Recombinant Adeno-Associated Virus-Mediated Expression for Large Transgenes, Using a Synthetic Promoter and Tandem Array Enhancers.Hum Gene Ther. 2015 Jun;26(6):334-46. doi: 10.1089/hum.2015.001. Epub 2015 Apr 20. Hum Gene Ther. 2015. PMID: 25763813 Free PMC article.
-
Answered and Unanswered Questions in Early-Stage Viral Vector Transduction Biology and Innate Primary Cell Toxicity for Ex-Vivo Gene Editing.Front Immunol. 2021 May 28;12:660302. doi: 10.3389/fimmu.2021.660302. eCollection 2021. Front Immunol. 2021. PMID: 34122418 Free PMC article. Review.
-
Viral Vectors, Animal Models, and Cellular Targets for Gene Therapy of Cystic Fibrosis Lung Disease.Hum Gene Ther. 2020 May;31(9-10):524-537. doi: 10.1089/hum.2020.013. Epub 2020 Apr 15. Hum Gene Ther. 2020. PMID: 32138545 Free PMC article. Review.
-
PTEN knockdown with the Y444F mutant AAV2 vector promotes axonal regeneration in the adult optic nerve.Neural Regen Res. 2018 Jan;13(1):135-144. doi: 10.4103/1673-5374.224381. Neural Regen Res. 2018. PMID: 29451218 Free PMC article.
-
Adeno-Associated Virus (AAV) Capsid Stability and Liposome Remodeling During Endo/Lysosomal pH Trafficking.Viruses. 2020 Jun 20;12(6):668. doi: 10.3390/v12060668. Viruses. 2020. PMID: 32575696 Free PMC article.
References
-
- Duan D, Yue Y, Yan Z, McCray PB, Engelhardt JF. Polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia. Hum. Gene Ther. 1998;9:2761–2776. - PubMed
-
- Zhong L, et al. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Hum. Gene Ther. 2004;15:1207–1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

