Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis
- PMID: 16443611
- PMCID: PMC1363909
- DOI: 10.1136/bmj.38719.435833.7C
Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis
Abstract
Objective: To evaluate the effects of hepatitis B vaccine and immunoglobulin in newborn infants of mothers positive for hepatitis B surface antigen.
Design: Systematic review and meta-analysis of randomised clinical trials.
Data sources: Electronic databases and hand searches.
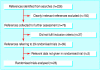
Review methods: Randomised clinical trials were assessed for methodological quality. Meta-analysis was undertaken on three outcomes: the relative risks of hepatitis B occurrence, antibody levels to hepatitis B surface antigen, and adverse events.
Results: 29 randomised clinical trials were identified, five of which were considered high quality. Only three trials reported inclusion of mothers negative for hepatitis B e antigen. Compared with placebo or no intervention, vaccination reduced the occurrence of hepatitis B (relative risk 0.28, 95% confidence interval 0.20 to 0.40; four trials). No significant difference in hepatitis B occurrence was found between recombinant vaccine and plasma derived vaccine (1.00, 0.71 to 1.42; four trials) and between high dose versus low dose vaccine (plasma derived vaccine 0.97, 0.55 to 1.68, three trials; recombinant vaccine 0.78, 0.31 to 1.94, one trial). Compared with placebo or no intervention, hepatitis B immunoglobulin or the combination of plasma derived vaccine and hepatitis B immunoglobulin reduced hepatitis B occurrence (immunoglobulin 0.50, 0.41 to 0.60, one trial; vaccine and immunoglobulin 0.08, 0.03 to 0.17, three trials). Compared with vaccine alone, vaccine plus hepatitis B immunoglobulin reduced hepatitis B occurrence (0.54, 0.41 to 0.73; 10 trials). Hepatitis B vaccine and hepatitis B immunoglobulin seem safe, but few trials reported adverse events.
Conclusion: Hepatitis B vaccine, hepatitis B immunoglobulin, and vaccine plus immunoglobulin prevent hepatitis B occurrence in newborn infants of mothers positive for hepatitis B surface antigen.
Figures



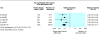
References
-
- Beasley RP, Hwang LY. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis 1984;4: 113-21. - PubMed
-
- Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975;292: 771-4. - PubMed
-
- Akhter S, Talukder MQ, Bhuiyan N, Chowdhury TA, Islam MN, Begum S. Hepatitis B virus infection in pregnant mothers and its transmission to infants. Indian J Pediatr 1992;59: 411-5. - PubMed
-
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. E antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976;294: 746-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
