ATP hydrolysis stimulates large length fluctuations in single actin filaments
- PMID: 16443647
- PMCID: PMC1414574
- DOI: 10.1529/biophysj.105.074211
ATP hydrolysis stimulates large length fluctuations in single actin filaments
Abstract
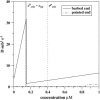
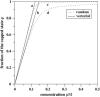
Polymerization dynamics of single actin filaments is investigated theoretically using a stochastic model that takes into account the hydrolysis of ATP-actin subunits, the geometry of actin filament tips, and the lateral interactions between the monomers as well as the processes at both ends of the polymer. Exact analytical expressions are obtained for the mean growth velocity, for the dispersion in the length fluctuations, and the nucleotide composition of the actin filaments. It is found that the ATP hydrolysis has a strong effect on dynamic properties of single actin filaments. At high concentrations of free actin monomers, the mean size of the unhydrolyzed ATP-cap is very large, and the dynamics is governed by association/dissociation of ATP-actin subunits. However, at low concentrations the size of the cap becomes finite, and the dissociation of ADP-actin subunits makes a significant contribution to overall dynamics. Actin filament length fluctuations reach a sharp maximum at the boundary between two dynamic regimes, and this boundary is always larger than the critical concentration for the actin filament's growth at the barbed end, assuming the sequential release of phosphate. Random and sequential mechanisms of hydrolysis are compared, and it is found that they predict qualitatively similar dynamic properties at low and high concentrations of free actin monomers with some deviations near the critical concentration. The possibility of attachment and detachment of oligomers in actin filament's growth is also discussed. Our theoretical approach is successfully applied to analyze the latest experiments on the growth and length fluctuations of individual actin filaments.
Figures



Similar articles
-
Self-assembly of actin monomers into long filaments: Brownian dynamics simulations.J Chem Phys. 2009 Jul 7;131(1):015102. doi: 10.1063/1.3159003. J Chem Phys. 2009. PMID: 19586123
-
Actin polymerization and ATP hydrolysis.Science. 1987 Oct 30;238(4827):638-44. doi: 10.1126/science.3672117. Science. 1987. PMID: 3672117
-
Actin polymerization kinetics, cap structure, and fluctuations.Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8543-8. doi: 10.1073/pnas.0501435102. Epub 2005 Jun 6. Proc Natl Acad Sci U S A. 2005. PMID: 15939882 Free PMC article.
-
Actin polymerization and ATP hydrolysis.Adv Biophys. 1990;26:51-73. doi: 10.1016/0065-227x(90)90007-g. Adv Biophys. 1990. PMID: 2082729 Review.
-
Molecular mechanisms controlling actin filament dynamics in nonmuscle cells.Annu Rev Biophys Biomol Struct. 2000;29:545-76. doi: 10.1146/annurev.biophys.29.1.545. Annu Rev Biophys Biomol Struct. 2000. PMID: 10940259 Review.
Cited by
-
Insights into Actin Polymerization and Nucleation Using a Coarse-Grained Model.Biophys J. 2020 Aug 4;119(3):553-566. doi: 10.1016/j.bpj.2020.06.019. Epub 2020 Jul 8. Biophys J. 2020. PMID: 32668234 Free PMC article.
-
Atomistic molecular dynamics simulations of tubulin heterodimers explain the motion of a microtubule.Eur Biophys J. 2021 Oct;50(7):927-940. doi: 10.1007/s00249-021-01553-1. Epub 2021 Jul 2. Eur Biophys J. 2021. PMID: 34215900 Free PMC article.
-
Design of active transport must be highly intricate: a possible role of myosin and Ena/VASP for G-actin transport in filopodia.Biophys J. 2010 Apr 21;98(8):1439-48. doi: 10.1016/j.bpj.2009.12.4325. Biophys J. 2010. PMID: 20409462 Free PMC article.
-
Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility.Dev Cell. 2016 Jan 25;36(2):201-14. doi: 10.1016/j.devcel.2015.12.024. Dev Cell. 2016. PMID: 26812019 Free PMC article.
-
The stochastic dynamics of filopodial growth.Biophys J. 2008 May 15;94(10):3839-52. doi: 10.1529/biophysj.107.123778. Epub 2008 Jan 30. Biophys J. 2008. PMID: 18234810 Free PMC article.
References
-
- Howard, J. 2001. Mechanics of Motor Proteins and Cytoskeleton. Sinauer Associates, Sunderland, MA.
-
- Bray, D. 2001. Cell Movements. From Molecules to Motility. Garland Publishing, New York.
-
- Pollard, T. D., and G. G. Borisy. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465. - PubMed
-
- Erickson, H. P., and E. T. O'Brien. 1992. Microtubule dynamic instability and GTP hydrolysis. Annu. Rev. Biophys. Biomol. Struct. 21:145–166. - PubMed
-
- Desai, A., and T. J. Mitchison. 1997. Microtubule polymerization dynamics. Annu. Rev. Cell Biol. 13:83–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

