Synthesis of voltage-sensitive optical signals: application to panoramic optical mapping
- PMID: 16443665
- PMCID: PMC1414570
- DOI: 10.1529/biophysj.105.076505
Synthesis of voltage-sensitive optical signals: application to panoramic optical mapping
Abstract
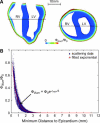
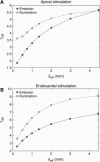
Fluorescent photon scattering is known to distort optical recordings of cardiac transmembrane potentials; however, this process is not well quantified, hampering interpretation of experimental data. This study presents a novel model, which accurately synthesizes fluorescent recordings over the irregular geometry of the rabbit ventricles. Using the model, the study aims to provide quantification of fluorescent signal distortion for different optical characteristics of the preparation and of the surrounding medium. A bi-domain representation of electrical activity is combined with finite element solutions to the photon diffusion equation simulating both the excitation and emission processes, along with physically realistic boundary conditions at the epicardium, which allow simulation of different experimental setups. We demonstrate that distortion in the optical signal as a result of fluorescent photon scattering is truly a three-dimensional phenomenon and depends critically upon the geometry of the preparation, the scattering properties of the tissue, the direction of wavefront propagation, and the specifics of the experimental setup. Importantly, we show that in an anatomically accurate model of ventricular geometry and fiber orientation, the morphology of the optical signal does not provide reliable information regarding the intramural direction of wavefront propagation. These findings underscore the potential of the new model in interpreting experimental data.
Figures



Comment in
-
What can we learn from the optically recorded epicardial action potential?Biophys J. 2006 Nov 15;91(10):3959-60. doi: 10.1529/biophysj.106.091835. Epub 2006 Aug 25. Biophys J. 2006. PMID: 16935958 Free PMC article.
References
-
- Loew, L. M. 2001. Optical mapping of cardiac excitation and arrhythmias. In Mechanisms and Principles of Voltage-Sensitive Fluorescence. Futura Publishing, Armonk, NY. 33–46.
-
- Efimov, I., V. Nikolski, and G. Salama. 2004. Optical imaging of the heart (review). Circ. Res. 94:21–33. - PubMed
-
- Ding, L., R. Splinter, and S. Knisley. 2001. Quantifying spatial localization of optical mapping using Monte Carlo simulations. IEEE Trans. Biomed. Eng. 48:1098–1107. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

