Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy
- PMID: 16443686
- PMCID: PMC1413632
- DOI: 10.1073/pnas.0507493103
Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy
Abstract
The p53 tumor suppressor retains its wild-type conformation and transcriptional activity in half of all human tumors, and its activation may offer a therapeutic benefit. However, p53 function could be compromised by defective signaling in the p53 pathway. Using a small-molecule MDM2 antagonist, nutlin-3, to probe downstream p53 signaling we find that the cell-cycle arrest function of the p53 pathway is preserved in multiple tumor-derived cell lines expressing wild-type p53, but many have a reduced ability to undergo p53-dependent apoptosis. Gene array analysis revealed attenuated expression of multiple apoptosis-related genes. Cancer cells with mdm2 gene amplification were most sensitive to nutlin-3 in vitro and in vivo, suggesting that MDM2 overexpression may be the only abnormality in the p53 pathway of these cells. Nutlin-3 also showed good efficacy against tumors with normal MDM2 expression, suggesting that many of the patients with wild-type p53 tumors may benefit from antagonists of the p53-MDM2 interaction.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
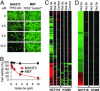

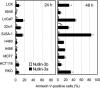

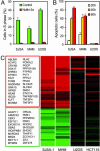

Comment in
-
Protein-protein interactions for cancer therapy.Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1659-60. doi: 10.1073/pnas.0510948103. Epub 2006 Feb 1. Proc Natl Acad Sci U S A. 2006. PMID: 16452164 Free PMC article. No abstract available.
References
-
- Levine A. J. Cell. 1997;88:323–331. - PubMed
-
- Vogelstein B., Lane D., Levine A. J. Nature. 2000;408:307–310. - PubMed
-
- Harris S. L., Levine A. J. Oncogene. 2005;24:2899–2908. - PubMed
-
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. Science. 1991;253:49–53. - PubMed
-
- Hainaut P., Hollstein M. Adv. Cancer Res. 2000;77:81–137. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

