Function and characterization of starch synthase I using mutants in rice
- PMID: 16443699
- PMCID: PMC1400558
- DOI: 10.1104/pp.105.071845
Function and characterization of starch synthase I using mutants in rice
Abstract
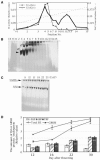
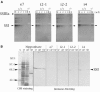
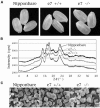
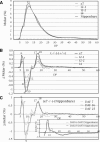
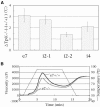
Four starch synthase I (SSI)-deficient rice (Oryza sativa) mutant lines were generated using retrotransposon Tos17 insertion. The mutants exhibited different levels of SSI activities and produced significantly lower amounts of SSI protein ranging from 0% to 20% of the wild type. The mutant endosperm amylopectin showed a decrease in chains with degree of polymerization (DP) 8 to 12 and an increase in chains with DP 6 to 7 and DP 16 to 19. The degree of change in amylopectin chain-length distribution was positively correlated with the extent of decrease in SSI activity in the mutants. The structural changes in the amylopectin increased the gelatinization temperature of endosperm starch. Chain-length analysis of amylopectin in the SSI band excised from native-polyacrylamide gel electrophoresis/SS activity staining gel showed that SSI preferentially synthesized DP 7 to 11 chains by elongating DP 4 to 7 short chains of glycogen or amylopectin. These results show that SSI distinctly generates DP 8 to 12 chains from short DP 6 to 7 chains emerging from the branch point in the A or B(1) chain of amylopectin. SSI seemingly functions from the very early through the late stage of endosperm development. Yet, the complete absence of SSI, despite being a major SS isozyme in the developing endosperm, had no effect on the size and shape of seeds and starch granules and the crystallinity of endosperm starch, suggesting that other SS enzymes are probably capable of partly compensating SSI function. In summary, this study strongly suggested that amylopectin chains are synthesized by the coordinated actions of SSI, SSIIa, and SSIIIa isoforms.
Figures
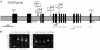


References
-
- Abel GJW, Springer F, Willmitzer L, Kossman J (1996) Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.). Plant J 10: 981–991 - PubMed
-
- Bertoft E (1991) Investigation of the fine structure of alpha-dextrins derived from amylopectin and their relation to the structure of waxy-maize starch. Carbohydr Res 212: 229–244 - PubMed
-
- Bertoft E (2004) On the nature of categories of chains in amylopectin and their connection to the super helix model. Carbohydr Polym 57: 211–224
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

