Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma
- PMID: 16443949
- PMCID: PMC1871923
- DOI: 10.1215/S1522851705000475
Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma
Abstract
Oligodeoxynucleotides containing CpG motifs (CpG ODNs) display a strong immunostimulating activity and drive the immune response toward the Th1 (T helper type 1) phenotype. These ODNs have shown promising efficacy in preclinical studies when injected locally in several cancer models. We conducted a phase 1 trial to define the safety profile of CpG-28, a phosphorothioate CpG ODN, administered intratumorally by convection-enhanced delivery in patients with recurrent glioblastoma. Cohorts of three to six patients were treated with escalating doses of CpG-28 (0.5-20 mg), and patients were observed for at least four months. Twenty-four patients entered the trial. All patients had previously been treated with radiotherapy, and most patients had received one or several types of chemotherapy. Median age was 58 years (range, 25-73) and median KPS was 80% (range, 60%-100%). Adverse effects possibly or probably related to the studied drug were moderate and consisted mainly in worsening of neurological conditions (four patients), fever above 38 degrees C that disappeared within a few days (five patients), and reversible grade 3 lymphopenia (seven patients). Only one patient experienced a dose-limiting toxicity. Preliminary evidence of activity was suggested by a minor response observed in two patients and an overall median survival of 7.2 months. In conclusion, CpG-28 was well tolerated at doses up to 20 mg per injection in patients with recurrent glioblastoma. Main side effects were limited to transient worsening of neurological condition and fever.
Figures
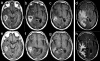


References
-
- Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. - PubMed
-
- Broaddus WC, Prabhu SS, Gillies GT, Neal J, Conrad WS, Chen ZJ, Fillmore H, Young HF. Distribution and stability of antisense phosphorothioate oligonucleotides in rodent brain following direct intraparenchymal controlled-rate infusion. J Neurosurg. 1998;88:734–742. - PubMed
-
- Carpentier AF, Chen L, Maltonti F, Delattre J.-Y. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429–5432. - PubMed
-
- Carpentier AF, Xie J, Mokhtari K, Delattre J.-Y. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. - PubMed
-
- Carpentier AF, Auf G, Delattre J.-Y. CpG-oligonucleotides for cancer immunotherapy: Review of the literature and potential applications in malignant glioma. Front Biosci. 2003;8:e115–e127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

