Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion
- PMID: 16444293
- PMCID: PMC1352158
- DOI: 10.1172/JCI24521
Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion
Abstract
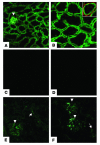
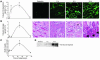
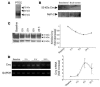
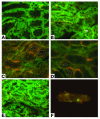
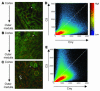
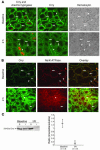
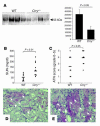
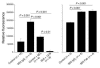
Ischemia/reperfusion (I/R) of several organs results in complement activation, but the kidney is unique in that activation after I/R occurs only via the alternative pathway. We hypothesized that selective activation of this pathway after renal I/R could occur either because of a loss of complement inhibition or from increased local synthesis of complement factors. We examined the relationship between renal complement activation after I/R and the levels and localization of intrinsic membrane complement inhibitors. We found that loss of polarity of complement receptor 1-related protein y (Crry) in the tubular epithelium preceded activation of the alternative pathway along the basolateral aspect of the tubular cells. Heterozygous gene-targeted mice that expressed lower amounts of Crry were more sensitive to ischemic injury. Furthermore, inhibition of Crry expressed by proximal tubular epithelial cells in vitro resulted in alternative pathway-mediated injury to the cells. Thus, altered expression of a complement inhibitor within the tubular epithelium appears to be a critical factor permitting activation of the alternative pathway of complement after I/R. Increased C3 mRNA and decreased factor H mRNA were also detected in the outer medulla after I/R, suggesting that altered synthesis of these factors might further contribute to complement activation in this location.
Figures


References
-
- Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J. Immunol. 2003;170:1517–1523. - PubMed
-
- De Vries B, et al. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J. Immunol. 2003;170:3883–3889. - PubMed
-
- Park P, et al. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am. J. Physiol. Renal Physiol. 2002;282:F352–F357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

