The diacylglycerol-binding protein alpha1-chimaerin regulates dendritic morphology
- PMID: 16446429
- PMCID: PMC1413663
- DOI: 10.1073/pnas.0510655103
The diacylglycerol-binding protein alpha1-chimaerin regulates dendritic morphology
Abstract
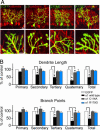
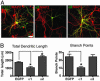
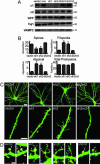
The morphological and functional differentiation of neuronal dendrites is controlled through transcriptional programs and cell-cell signaling. Synaptic activity is thought to play an important role in the maturation of dendritic arbors, but the signaling pathways that couple neuronal activity and morphological changes in dendrites are not well understood. We explored the function of alpha1-chimaerin, a neuronal diacylglycerol-binding protein with a Rho GTPase-activating protein domain that inactivates Rac1. We find that stimulation of phospholipase Cbeta-coupled cell surface receptors recruits alpha1-chimaerin to the plasma membrane of cultured hippocampal neurons. We further show that alpha1-chimaerin protein levels are controlled by synaptic activity and that increased alpha1-chimaerin expression results in the pruning of dendritic spines and branches. This pruning activity requires both the diacylglycerol-binding and Rac GTPase-activating protein activity of alpha1-chimaerin. Suppression of alpha1-chimaerin expression resulted in increased process growth from the dendritic shaft and from spine heads. Our data suggest that alpha1-chimaerin is an activity-regulated Rho GTPase regulator that is activated by phospholipase Cbeta-coupled cell surface receptors and contributes to pruning of dendritic arbors.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
-
- Bonhoeffer T., Yuste R. Neuron. 2002;35:1019–1027. - PubMed
-
- Wong R. O., Ghosh A. Nat. Rev. Neurosci. 2002;3:803–812. - PubMed
-
- Niell C. M., Meyer M. P., Smith S. J. Nat. Neurosci. 2004;7:254–260. - PubMed
-
- Sin W. C., Haas K., Ruthazer E. S., Cline H. T. Nature. 2002;419:475–480. - PubMed
-
- Hua J. Y., Smear M. C., Baier H., Smith S. J. Nature. 2005;434:1022–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

