The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone
- PMID: 16446434
- PMCID: PMC1413654
- DOI: 10.1073/pnas.0510410103
The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone
Abstract
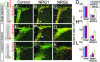
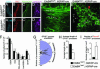
Coordinated regulation of neuronal progenitor differentiation in the subventricular zone (SVZ) is a fundamental feature of adult neurogenesis. However, the molecular control of this process remains mostly undeciphered. Here, we investigate the role of neuregulins (NRGs) in this process and show that a NRG receptor, ErbB4, is primarily expressed by polysialylated neural cell adhesion molecule immature neuroblasts but is also detected in a subset of GFAP+ astroglial cells, ependymal cells, and Dlx2+ precursors in the SVZ. Of the NRG ligands, both NRG1 and -2 are expressed by immature polysialylated neural cell adhesion molecule neuroblasts in the SVZ. NRG2 is also expressed by some of the GFAP+ putative stem cells lining the ventricles. Infusion of exogenous NRG1 leads to rapid aggregation of Dlx2+ cells in the SVZ and affects the initiation and maintenance of organized neuroblast migration from the SVZ toward the olfactory bulb. In contrast, the infusion of NRG2 increased the number of Sox2 and GFAP+ precursors in the SVZ. An outcome of this NRG2 effect is an increase in the number of newly generated migrating neuroblasts in the rostral migratory stream and GABAergic interneurons in the olfactory bulb. The analysis of conditional null mice that lack NRG receptor, ErbB4, in the nervous system revealed that the observed activities of NRG2 require ErbB4 activation. These results indicate that different NRG ligands affect distinct populations of differentiating neural precursors in the neurogenic regions of the mature forebrain. Furthermore, these studies identify NRG2 as a factor capable of promoting SVZ proliferation, leading to the formation of new neurons in vivo.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures





Similar articles
-
Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain.Nat Neurosci. 2004 Dec;7(12):1319-28. doi: 10.1038/nn1345. Epub 2004 Nov 7. Nat Neurosci. 2004. PMID: 15543145
-
EphA4 Regulates Neuroblast and Astrocyte Organization in a Neurogenic Niche.J Neurosci. 2017 Mar 22;37(12):3331-3341. doi: 10.1523/JNEUROSCI.3738-16.2017. Epub 2017 Mar 3. J Neurosci. 2017. PMID: 28258169 Free PMC article.
-
Neuregulin repellent signaling via ErbB4 restricts GABAergic interneurons to migratory paths from ganglionic eminence to cortical destinations.Neural Dev. 2012 Feb 29;7:10. doi: 10.1186/1749-8104-7-10. Neural Dev. 2012. PMID: 22376909 Free PMC article.
-
Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review.
-
Extracellular signals controlling neuroblast migration in the postnatal brain.Adv Exp Med Biol. 2014;800:149-80. doi: 10.1007/978-94-007-7687-6_9. Adv Exp Med Biol. 2014. PMID: 24243105 Review.
Cited by
-
Targeted deletion of the ERK5 MAP kinase impairs neuronal differentiation, migration, and survival during adult neurogenesis in the olfactory bulb.PLoS One. 2013 Apr 22;8(4):e61948. doi: 10.1371/journal.pone.0061948. Print 2013. PLoS One. 2013. PMID: 23630619 Free PMC article.
-
Seasonal remodeling of the progenitor pool and its distribution in the ewe mediobasal hypothalamus.Cell Tissue Res. 2023 Jun;392(3):745-761. doi: 10.1007/s00441-023-03745-x. Epub 2023 Feb 16. Cell Tissue Res. 2023. PMID: 36795154
-
Protein Kinase C Inhibition Mediates Neuroblast Enrichment in Mechanical Brain Injuries.Front Cell Neurosci. 2018 Nov 27;12:462. doi: 10.3389/fncel.2018.00462. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30542270 Free PMC article.
-
TGF-β1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo.Front Cell Neurosci. 2014 Nov 21;8:393. doi: 10.3389/fncel.2014.00393. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25484855 Free PMC article.
-
Impaired APP activity and altered Tau splicing in embryonic stem cell-derived astrocytes obtained from an APPsw transgenic minipig.Dis Model Mech. 2015 Oct 1;8(10):1265-78. doi: 10.1242/dmm.019489. Epub 2015 Aug 6. Dis Model Mech. 2015. PMID: 26398935 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

