Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen
- PMID: 16446444
- PMCID: PMC1413641
- DOI: 10.1073/pnas.0508983103
Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen
Abstract
Infections caused by the obligate intracellular pathogen Chlamydia trachomatis have a marked impact on human health. C. trachomatis serovariants are the leading cause of bacterial sexually transmitted disease and infectious preventable blindness. Despite decades of effort, there is no practical vaccine against C. trachomatis diseases. Here we report that all C. trachomatis reference serotypes responsible for sexually transmitted disease and blinding trachoma synthesize a highly conserved surface-exposed antigen termed polymorphic membrane protein D (PmpD). We show that Ab specific to PmpD are neutralizing in vitro. We also present evidence that Ab against serovariable-neutralizing targets, such as the major outer membrane protein, block PmpD neutralization. This finding suggests that a decoy-like immune evasion strategy may be active in vivo whereby immunodominant type-specific surface antigens block the neutralizing ability of species-common PmpD Ab. Collectively, these results show that PmpD is a previously uncharacterized C. trachomatis species-common pan-neutralizing target. Moreover, a vaccine protocol using recombinant PmpD to elicit neutralizing Ab in the absence of immunodominant type-specific Ab might be highly efficacious and surpass the level of protection achieved through natural immunity.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
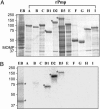
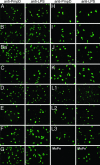
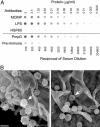
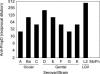
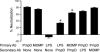

References
-
- Division of STD Prevention. Sexually Transmitted Disease Surveillance 1997 (Centers Dis. Control Prev., Atlanta) 1998.
-
- World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates. Geneva: World Health Org.; 2001. pp. 1–43.
-
- Westrom L., Joesoef R., Reynolds G., Hagdu A., Thompson S. E. Sex. Transm. Dis. 1992;19:185–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

