Odorant responses of olfactory sensory neurons expressing the odorant receptor MOR23: a patch clamp analysis in gene-targeted mice
- PMID: 16446455
- PMCID: PMC1413638
- DOI: 10.1073/pnas.0508491103
Odorant responses of olfactory sensory neurons expressing the odorant receptor MOR23: a patch clamp analysis in gene-targeted mice
Abstract
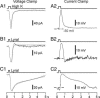
A glomerulus in the mammalian olfactory bulb receives axonal inputs from olfactory sensory neurons (OSNs) that express the same odorant receptor (OR). Glomeruli are generally thought to represent functional units of olfactory coding, but there are no data on the electrophysiological properties of OSNs that express the same endogenous OR. Here, using patch clamp recordings in an intact epithelial preparation, we directly measured the transduction currents and receptor potentials from the dendritic knobs of mouse OSNs that express the odorant receptor MOR23 along with the green fluorescent protein. All of the 53 cells examined responded to lyral, a known ligand for MOR23. There were profound differences in response kinetics, particularly in the deactivation phase. The cells were very sensitive to lyral, with some cells responding to as little as 10 nM. The dynamic range was unexpectedly broad, with threshold and saturation in individual cells often covering three log units of lyral concentration. The potential causes and biological significance of this cellular heterogeneity are discussed. Patch clamp recording from OSNs that express a defined OR provides a powerful approach to investigate the sensory inputs to individual glomeruli.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


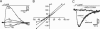

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

