Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency
- PMID: 16446459
- PMCID: PMC1413655
- DOI: 10.1073/pnas.0510452103
Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency
Abstract
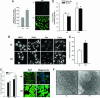
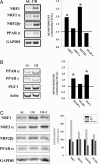
Age-related accumulation of cellular damage and death has been linked to oxidative stress. Calorie restriction (CR) is the most robust, nongenetic intervention that increases lifespan and reduces the rate of aging in a variety of species. Mechanisms responsible for the antiaging effects of CR remain uncertain, but reduction of oxidative stress within mitochondria remains a major focus of research. CR is hypothesized to decrease mitochondrial electron flow and proton leaks to attenuate damage caused by reactive oxygen species. We have focused our research on a related, but different, antiaging mechanism of CR. Specifically, using both in vivo and in vitro analyses, we report that CR reduces oxidative stress at the same time that it stimulates the proliferation of mitochondria through a peroxisome proliferation-activated receptor coactivator 1 alpha signaling pathway. Moreover, mitochondria under CR conditions show less oxygen consumption, reduce membrane potential, and generate less reactive oxygen species than controls, but remarkably they are able to maintain their critical ATP production. In effect, CR can induce a peroxisome proliferation-activated receptor coactivator 1 alpha-dependent increase in mitochondria capable of efficient and balanced bioenergetics to reduce oxidative stress and attenuate age-dependent endogenous oxidative damage.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Nicholls D. G. Aging Cell. 2004;3:35–40. - PubMed
-
- Salvioli S., Bonafe M., Capri M., Monti D., Franceschi C. FEBS Lett. 2001;492:9–13. - PubMed
-
- Lenaz G., D’Aurelio M., Merlo Pich M., Genova M. L., Ventura B., Bovina C., Formiggini G., Parenti Castelli G. Biochim. Biophys. Acta. 2000;1459:397–404. - PubMed
-
- Sohal R. S., Svensson I., Brunk U. T. Mech. Ageing Dev. 1990;53:209–215. - PubMed

