Identification of a disulfide bridge linking the fourth and the seventh extracellular loops of the Na+/glucose cotransporter
- PMID: 16446504
- PMCID: PMC2151483
- DOI: 10.1085/jgp.200509439
Identification of a disulfide bridge linking the fourth and the seventh extracellular loops of the Na+/glucose cotransporter
Abstract
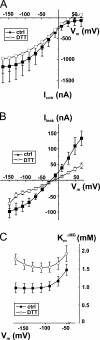
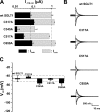
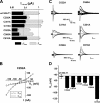
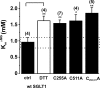
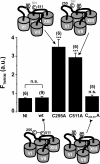
The Na+/glucose cotransporter (SGLT1) is an archetype for the SLC5 family, which is comprised of Na+-coupled transporters for sugars, myo-inositol, choline, and organic anions. Application of the reducing agent dithriothreitol (DTT, 10 mM) to oocytes expressing human SGLT1 affects the protein's presteady-state currents. Integration of these currents at different membrane potentials (Vm) produces a Q-V curve, whose form was shifted by +25 mV due to DTT. The role of the 15 endogenous cysteine residues was investigated by expressing SGLT1 constructs, each bearing a single mutation for an individual cysteine, in Xenopus oocytes, using two-microelectrode voltage-clamp electrophysiology and fluorescent labeling. 12 of the 15 mutants were functional and could be separated into three distinct groups based on the effect of the mutation on the Q-V curve: four mutants did not perturb the transferred charge, six mutants shifted the Q-V curve towards negative potentials, and two mutants (C255A and C511A) produced a shift in the positive direction that was identical to the shift produced by DTT on the wild-type (wt) SGLT1. The double mutant C(255,511)A confirms that the effects of each single mutant on the Q-V curve were not additive. With respect to wt SGLT1, the apparent affinities for alpha-methylglucose (alphaMG) were increased in a similar manner for the single mutants C255A and C511A, the double mutant C(255,511)A as well as for wt SGLT1 treated with DTT. When exposed to a maleimide-based fluorescent probe, wt SGLT1 was not significantly labeled but mutants C255A and C511A could be clearly labeled, indicating an accessible cysteine residue. These residues are presumed to be C511 and C255, respectively, as the double mutant C(255,511)A could not be labeled. These results strongly support the hypothesis that C255 and C511 form a disulfide bridge in human SGLT1 and that this disulfide bridge is involved in the conformational change of the free carrier.
Figures



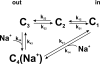
Similar articles
-
Effect of substrate on the pre-steady-state kinetics of the Na(+)/glucose cotransporter.Biophys J. 2007 Jan 15;92(2):461-72. doi: 10.1529/biophysj.106.092296. Epub 2006 Oct 27. Biophys J. 2007. PMID: 17071656 Free PMC article.
-
Voltage-clamp fluorometry in the local environment of the C255-C511 disulfide bridge of the Na+/glucose cotransporter.Biophys J. 2007 Apr 1;92(7):2403-11. doi: 10.1529/biophysj.106.097964. Epub 2007 Jan 5. Biophys J. 2007. PMID: 17208964 Free PMC article.
-
Conformational dynamics of hSGLT1 during Na+/glucose cotransport.J Gen Physiol. 2006 Dec;128(6):701-20. doi: 10.1085/jgp.200609643. J Gen Physiol. 2006. PMID: 17130520 Free PMC article.
-
The actual ionic nature of the leak current through the Na+/glucose cotransporter SGLT1.Biophys J. 2010 Jan 20;98(2):231-9. doi: 10.1016/j.bpj.2009.10.015. Biophys J. 2010. PMID: 20338844 Free PMC article.
-
A biophysical glance at the outer surface of the membrane transporter SGLT1.Biochim Biophys Acta. 2011 Jan;1808(1):1-18. doi: 10.1016/j.bbamem.2010.07.028. Epub 2010 Aug 6. Biochim Biophys Acta. 2011. PMID: 20692230 Review.
Cited by
-
Identification of a disulfide bridge essential for transport function of the human proton-coupled amino acid transporter hPAT1.J Biol Chem. 2009 Aug 14;284(33):22123-22132. doi: 10.1074/jbc.M109.023713. Epub 2009 Jun 23. J Biol Chem. 2009. PMID: 19549785 Free PMC article.
-
Effect of substrate on the pre-steady-state kinetics of the Na(+)/glucose cotransporter.Biophys J. 2007 Jan 15;92(2):461-72. doi: 10.1529/biophysj.106.092296. Epub 2006 Oct 27. Biophys J. 2007. PMID: 17071656 Free PMC article.
-
Structural basis of inhibition of the human SGLT2-MAP17 glucose transporter.Nature. 2022 Jan;601(7892):280-284. doi: 10.1038/s41586-021-04212-9. Epub 2021 Dec 8. Nature. 2022. PMID: 34880493
-
Effects on conformational states of the rabbit sodium/glucose cotransporter through modulation of polarity and charge at glutamine 457.Biophys J. 2009 Jan;96(2):748-60. doi: 10.1016/j.bpj.2008.09.045. Biophys J. 2009. PMID: 19167319 Free PMC article.
-
Distribution of glucose transporters in renal diseases.J Biomed Sci. 2017 Aug 31;24(1):64. doi: 10.1186/s12929-017-0371-7. J Biomed Sci. 2017. PMID: 28854935 Free PMC article. Review.
References
-
- Chen, J.G., S. Liu-Chen, and G. Rudnick. 1997. External cysteine residues in the serotonin transporter. Biochemistry. 36:1479–1486. - PubMed

