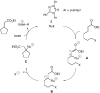
Umpolung of Michael acceptors catalyzed by N-heterocyclic carbenes
- PMID: 16448117
- PMCID: PMC2553003
- DOI: 10.1021/ja058222q
Umpolung of Michael acceptors catalyzed by N-heterocyclic carbenes
Abstract
N-Heterocyclic carbenes can catalyze beta-alkylations of a range of alpha,beta-unsaturated esters, amides, and nitriles that bear pendant leaving groups to form a variety of ring sizes. In this process, the nucleophilic catalyst transiently transforms the normally electrophilic beta carbon into a nucleophilic site through an unanticipated addition-tautomerization sequence.
Figures
Similar articles
-
Scope of the asymmetric intramolecular stetter reaction catalyzed by chiral nucleophilic triazolinylidene carbenes.J Org Chem. 2008 Mar 21;73(6):2033-40. doi: 10.1021/jo702313f. Epub 2008 Feb 27. J Org Chem. 2008. PMID: 18302407 Free PMC article.
-
A highly enantioselective intramolecular Michael reaction catalyzed by N-heterocyclic carbenes.Angew Chem Int Ed Engl. 2007;46(17):3107-10. doi: 10.1002/anie.200605235. Angew Chem Int Ed Engl. 2007. PMID: 17366506 Free PMC article. No abstract available.
-
Small-molecule N-heterocyclic-carbene-containing olefin-metathesis catalysts for use in water.Angew Chem Int Ed Engl. 2007;46(27):5152-5. doi: 10.1002/anie.200701258. Angew Chem Int Ed Engl. 2007. PMID: 17549784 No abstract available.
-
Organocatalysis by N-heterocyclic carbenes.Chem Rev. 2007 Dec;107(12):5606-55. doi: 10.1021/cr068372z. Epub 2007 Oct 23. Chem Rev. 2007. PMID: 17956132 Review. No abstract available.
-
[Development of C-C bond formation and asymmetric reactions catalyzed by N-heterocyclic carbenes].Yakugaku Zasshi. 2008 Aug;128(8):1179-85. doi: 10.1248/yakushi.128.1179. Yakugaku Zasshi. 2008. PMID: 18670183 Review. Japanese.
Cited by
-
NHC Catalysis for Umpolung Pyridinium Alkylation via Deoxy-Breslow Intermediates.Angew Chem Int Ed Engl. 2022 Apr 4;61(15):e202117524. doi: 10.1002/anie.202117524. Epub 2022 Feb 18. Angew Chem Int Ed Engl. 2022. PMID: 35103381 Free PMC article.
-
5-Benzyl-2-phenyl-6,8-dihydro-5H-1,2,4-triazolo[3,4-c][1,4]oxazin-2-ium hexa-fluoridophosphate.Acta Crystallogr Sect E Struct Rep Online. 2009 May 20;65(Pt 6):o1328. doi: 10.1107/S1600536809018005. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21583182 Free PMC article.
-
N-heterocyclic carbene-catalyzed conjugate additions of alcohols.J Am Chem Soc. 2010 Sep 29;132(38):13179-81. doi: 10.1021/ja1061196. J Am Chem Soc. 2010. PMID: 20809579 Free PMC article.
-
One Amine-3 Tasks: Reductive Coupling of Imines with Olefins in Batch and Flow.Chemistry. 2020 Jan 27;26(6):1363-1367. doi: 10.1002/chem.201904483. Epub 2020 Jan 21. Chemistry. 2020. PMID: 31777987 Free PMC article.
-
Carbene catalysts.Top Curr Chem. 2010;291:77-144. doi: 10.1007/978-3-642-02815-1_18. Top Curr Chem. 2010. PMID: 21494949 Free PMC article. Review.
References
-
-
For leading references, see: Powell DA, Maki T, Fu GC. J. Am. Chem. Soc. 2005;127:510–511.
-
-
-
For overviews of processes catalyzed by nucleophilic carbenes, see: Zeitler K. Angew. Chem., Int. Ed. 2005;44:7506–7510. Nair V, Bindu S, Sreekumar V. Angew. Chem., Int. Ed. 2004;43:5130–5135.
-
-
-
For a review of processes catalyzed by nucleophilic phosphines, see: Methot JL, Roush WR. Adv. Synth. Catal. 2004;346:1035–1050.
-
-
-
For precedent in a more highly activated substrate, see: Enders D, Breuer K, Teles JH, Ebel KJ. Prakt. Chem. 1997;339:397–399.
-
-
- Seebach D. Angew. Chem., Int. Ed. Engl. 1979;18:239–258.
- Hase TA, editor. Umpoled Synthons. Wiley; New York: 1987.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources