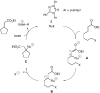
Umpolung of Michael acceptors catalyzed by N-heterocyclic carbenes
- PMID: 16448117
- PMCID: PMC2553003
- DOI: 10.1021/ja058222q
Umpolung of Michael acceptors catalyzed by N-heterocyclic carbenes
Abstract
N-Heterocyclic carbenes can catalyze beta-alkylations of a range of alpha,beta-unsaturated esters, amides, and nitriles that bear pendant leaving groups to form a variety of ring sizes. In this process, the nucleophilic catalyst transiently transforms the normally electrophilic beta carbon into a nucleophilic site through an unanticipated addition-tautomerization sequence.
Figures
References
-
-
For leading references, see: Powell DA, Maki T, Fu GC. J. Am. Chem. Soc. 2005;127:510–511.
-
-
-
For overviews of processes catalyzed by nucleophilic carbenes, see: Zeitler K. Angew. Chem., Int. Ed. 2005;44:7506–7510. Nair V, Bindu S, Sreekumar V. Angew. Chem., Int. Ed. 2004;43:5130–5135.
-
-
-
For a review of processes catalyzed by nucleophilic phosphines, see: Methot JL, Roush WR. Adv. Synth. Catal. 2004;346:1035–1050.
-
-
-
For precedent in a more highly activated substrate, see: Enders D, Breuer K, Teles JH, Ebel KJ. Prakt. Chem. 1997;339:397–399.
-
-
- Seebach D. Angew. Chem., Int. Ed. Engl. 1979;18:239–258.
- Hase TA, editor. Umpoled Synthons. Wiley; New York: 1987.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources