Cellular cholesterol controls TRPC3 function: evidence from a novel dominant-negative knockdown strategy
- PMID: 16448384
- PMCID: PMC1449990
- DOI: 10.1042/BJ20051246
Cellular cholesterol controls TRPC3 function: evidence from a novel dominant-negative knockdown strategy
Abstract
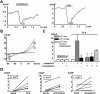
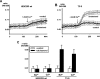
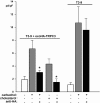
TRPC3 (canonical transient receptor potential protein 3) has been suggested to be a component of cation channel complexes that are targeted to cholesterol-rich lipid membrane microdomains. In the present study, we investigated the potential role of membrane cholesterol as a regulator of cellular TRPC3 conductances. Functional experiments demonstrated that cholesterol loading activates a non-selective cation conductance and a Ca2+ entry pathway in TRPC3-overexpressing cells but not in wild-type HEK-293 (human embryonic kidney 293) cells. The cholesterol-induced membrane conductance exhibited a current-to-voltage relationship similar to that observed upon PLC (phospholipase C)-dependent activation of TRPC3 channels. Nonetheless, the cholesterol-activated conductance lacked negative modulation by extracellular Ca2+, a typical feature of agonist-activated TRPC3 currents. Involvement of TRPC3 in the cholesterol-dependent membrane conductance was further corroborated by a novel dominant-negative strategy for selective blockade of TRPC3 channel activity. Expression of a TRPC3 mutant, which contained a haemagglutinin epitope tag in the second extracellular loop, conferred antibody sensitivity to both the classical PLC-activated as well as the cholesterol-activated conductance in TRPC3-expressing cells. Moreover, cholesterol loading as well as PLC stimulation was found to increase surface expression of TRPC3. Promotion of TRPC3 membrane expression by cholesterol was persistent over 30 min, while PLC-mediated enhancement of plasma membrane expression of TRPC3 was transient in nature. We suggest the cholesterol content of the plasma membrane as a determinant of cellular TRPC3 activity and provide evidence for cholesterol dependence of TRPC3 surface expression.
Figures
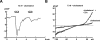

Similar articles
-
Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex.Cardiovasc Res. 2007 Jan 1;73(1):111-9. doi: 10.1016/j.cardiores.2006.10.016. Epub 2006 Oct 26. Cardiovasc Res. 2007. PMID: 17129578
-
Overexpression of TRPC3 reduces the content of intracellular calcium stores in HEK-293 cells.J Cell Physiol. 2008 Jul;216(1):245-52. doi: 10.1002/jcp.21396. J Cell Physiol. 2008. PMID: 18431718
-
Protein kinase C can inhibit TRPC3 channels indirectly via stimulating protein kinase G.J Cell Physiol. 2006 May;207(2):315-21. doi: 10.1002/jcp.20567. J Cell Physiol. 2006. PMID: 16331690
-
TRPC channels: integrators of multiple cellular signals.Handb Exp Pharmacol. 2007;(179):575-91. doi: 10.1007/978-3-540-34891-7_34. Handb Exp Pharmacol. 2007. PMID: 17217080 Review.
-
Signalling mechanisms for TRPC3 channels.Novartis Found Symp. 2004;258:123-33; discussion 133-9, 155-9, 263-6. Novartis Found Symp. 2004. PMID: 15104179 Review.
Cited by
-
TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties.Int J Mol Sci. 2019 Jan 16;20(2):371. doi: 10.3390/ijms20020371. Int J Mol Sci. 2019. PMID: 30654572 Free PMC article. Review.
-
TRPC channel lipid specificity and mechanisms of lipid regulation.Cell Calcium. 2009 Jun;45(6):583-8. doi: 10.1016/j.ceca.2009.02.006. Epub 2009 Mar 25. Cell Calcium. 2009. PMID: 19324410 Free PMC article. Review.
-
TRPC channels function independently of STIM1 and Orai1.J Physiol. 2009 May 15;587(Pt 10):2275-98. doi: 10.1113/jphysiol.2009.170431. Epub 2009 Mar 30. J Physiol. 2009. PMID: 19332491 Free PMC article.
-
TRP Channels in Tumoral Processes Mediated by Oxidative Stress and Inflammation.Antioxidants (Basel). 2023 Jun 23;12(7):1327. doi: 10.3390/antiox12071327. Antioxidants (Basel). 2023. PMID: 37507867 Free PMC article. Review.
-
Lipid raft segregation modulates TRPM8 channel activity.J Biol Chem. 2009 Apr 3;284(14):9215-24. doi: 10.1074/jbc.M807228200. Epub 2009 Jan 27. J Biol Chem. 2009. PMID: 19176480 Free PMC article.
References
-
- Vennekens R., Voets T., Bindels R. J., Droogmans G., Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. - PubMed
-
- Nilius B., Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001;81:1415–1459. - PubMed
-
- Sergeeva O. A., Korotkova T. M., Scherer A., Brown R. E., Haas H. L. Co-expression of non-selective cation channels of the transient receptor potential canonical family in central aminergic neurones. J. Neurochem. 2003;85:1547–1552. - PubMed
-
- Schilling W. P. TRP proteins: novel therapeutic targets for regional blood pressure control? Circ. Res. 2001;88:256–259. - PubMed
-
- Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Science STKE 2001. 2001:RE1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

