Reinvestigation of the dysbindin subunit of BLOC-1 (biogenesis of lysosome-related organelles complex-1) as a dystrobrevin-binding protein
- PMID: 16448387
- PMCID: PMC1462696
- DOI: 10.1042/BJ20051965
Reinvestigation of the dysbindin subunit of BLOC-1 (biogenesis of lysosome-related organelles complex-1) as a dystrobrevin-binding protein
Abstract
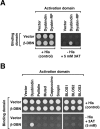
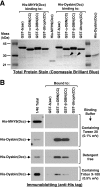
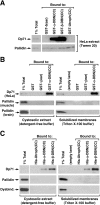
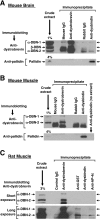
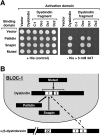
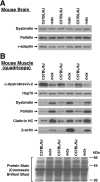
Dysbindin was identified as a dystrobrevin-binding protein potentially involved in the pathogenesis of muscular dystrophy. Subsequently, genetic studies have implicated variants of the human dysbindin-encoding gene, DTNBP1, in the pathogeneses of Hermansky-Pudlak syndrome and schizophrenia. The protein is a stable component of a multisubunit complex termed BLOC-1 (biogenesis of lysosome-related organelles complex-1). In the present study, the significance of the dystrobrevin-dysbindin interaction for BLOC-1 function was examined. Yeast two-hybrid analyses, and binding assays using recombinant proteins, demonstrated direct interaction involving coiled-coil-forming regions in both dysbindin and the dystrobrevins. However, recombinant proteins bearing the coiled-coil-forming regions of the dystrobrevins failed to bind endogenous BLOC-1 from HeLa cells or mouse brain or muscle, under conditions in which they bound the Dp71 isoform of dystrophin. Immunoprecipitation of endogenous dysbindin from brain or muscle resulted in robust co-immunoprecipitation of the pallidin subunit of BLOC-1 but no specific co-immunoprecipitation of dystrobrevin isoforms. Within BLOC-1, dysbindin is engaged in interactions with three other subunits, named pallidin, snapin and muted. We herein provide evidence that the same 69-residue region of dysbindin that is sufficient for dystrobrevin binding in vitro also contains the binding sites for pallidin and snapin, and at least part of the muted-binding interface. Functional, histological and immunohistochemical analyses failed to detect any sign of muscle pathology in BLOC-1-deficient, homozygous pallid mice. Taken together, these results suggest that dysbindin assembled into BLOC-1 is not a physiological binding partner of the dystrobrevins, likely due to engagement of its dystrobrevin-binding region in interactions with other subunits.
Figures


References
-
- Blake D. J., Weir A., Newey S. E., Davies K. E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. - PubMed
-
- Albrecht D. E., Froehner S. C. Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals. 2002;11:123–129. - PubMed
-
- Durbeej M., Campbell K. P. Muscular dystrophies involving the dystrophin–glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 2002;12:349–361. - PubMed
-
- Blake D. J. Dystrobrevin dynamics in muscle-cell signaling: a possible target for therapeutic intervention in Duchenne muscular dystrophy? Neuromuscul. Disord. 2002;12:S110–S117. - PubMed
-
- Grady R. M., Grange R. W., Lau K. S., Maimone M. M., Nichol M. C., Stull J. T., Sanes J. R. Role for α-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat. Cell Biol. 1999;1:215–220. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

