P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts
- PMID: 16449192
- PMCID: PMC2063651
- DOI: 10.1083/jcb.200505175
P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts
Abstract
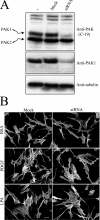
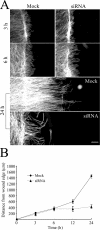
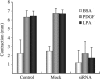
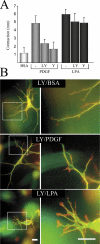
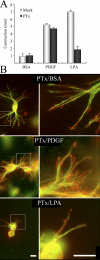
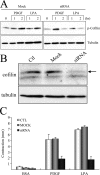
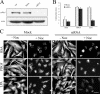
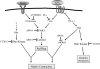
Fibroblast three-dimensional collagen matrix culture provides a tissue-like model that can be used to analyze cell form and function. The physiological agonists platelet-derived growth factor (PDGF) and lysophosphatidic acid (LPA) both stimulate human fibroblasts to contract floating collagen matrices. In this study, we show that the PDGF and LPA signaling pathways required for matrix contraction converge on p21-activated kinase 1 (PAK1) and its downstream effector cofilin1 and that contraction depends on cellular ruffling activity, rather than on the protrusion and retraction of cellular dendritic extensions. We also show that, depending on the agonist, different Rho effectors cooperate with PAK1 to regulate matrix contraction, Rho kinase in the case of PDGF and mDia1 in the case of LPA. These findings establish a unified framework for understanding the cell signaling pathways involved in fibroblast contraction of floating collagen matrices.
Figures


Similar articles
-
LPA-stimulated fibroblast contraction of floating collagen matrices does not require Rho kinase activity or retraction of fibroblast extensions.Exp Cell Res. 2003 Sep 10;289(1):86-94. doi: 10.1016/s0014-4827(03)00254-4. Exp Cell Res. 2003. PMID: 12941607
-
Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices.J Biol Chem. 1999 Jan 8;274(2):918-23. doi: 10.1074/jbc.274.2.918. J Biol Chem. 1999. PMID: 9873032
-
Different molecular motors mediate platelet-derived growth factor and lysophosphatidic acid-stimulated floating collagen matrix contraction.J Biol Chem. 2003 Nov 28;278(48):47707-12. doi: 10.1074/jbc.M306228200. Epub 2003 Sep 21. J Biol Chem. 2003. PMID: 14504290
-
The RHOad less traveled: the myosin phosphorylation-independent path from Rho kinase to cell contraction. Focus on "Rho kinase mediates serum-induced contraction in fibroblast fibers independent of myosin LC20 phosphorylation".Am J Physiol Cell Physiol. 2003 Mar;284(3):C596-8. doi: 10.1152/ajpcell.00530.2002. Am J Physiol Cell Physiol. 2003. PMID: 12556358 Review. No abstract available.
-
Fibroblast mechanics in 3D collagen matrices.Adv Drug Deliv Rev. 2007 Nov 10;59(13):1299-305. doi: 10.1016/j.addr.2007.08.006. Epub 2007 Aug 14. Adv Drug Deliv Rev. 2007. PMID: 17825456 Free PMC article. Review.
Cited by
-
Polydopamine-assisted PDGF-BB immobilization on PLGA fibrous substrate enhances wound healing via regulating anti-inflammatory and cytokine secretion.PLoS One. 2020 Sep 29;15(9):e0239366. doi: 10.1371/journal.pone.0239366. eCollection 2020. PLoS One. 2020. PMID: 32991599 Free PMC article.
-
Collagen fibril flow and tissue translocation coupled to fibroblast migration in 3D collagen matrices.Mol Biol Cell. 2008 May;19(5):2051-8. doi: 10.1091/mbc.e07-09-0930. Epub 2008 Mar 5. Mol Biol Cell. 2008. PMID: 18321993 Free PMC article.
-
Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling.PLoS One. 2010 Dec 30;5(12):e15940. doi: 10.1371/journal.pone.0015940. PLoS One. 2010. PMID: 21209852 Free PMC article.
-
Clinical application of growth factors and cytokines in wound healing.Wound Repair Regen. 2014 Sep-Oct;22(5):569-78. doi: 10.1111/wrr.12205. Wound Repair Regen. 2014. PMID: 24942811 Free PMC article. Review.
-
Small cytoskeleton-associated molecule, fibroblast growth factor receptor 1 oncogene partner 2/wound inducible transcript-3.0 (FGFR1OP2/wit3.0), facilitates fibroblast-driven wound closure.Am J Pathol. 2010 Jan;176(1):108-21. doi: 10.2353/ajpath.2010.090256. Epub 2009 Dec 3. Am J Pathol. 2010. PMID: 19959814 Free PMC article.
References
-
- Abe, M., C.H. Ho, K.E. Kamm, and F. Grinnell. 2003. Different molecular motors mediate platelet-derived growth factor and lysophosphatidic acid-stimulated floating collagen matrix contraction. J. Biol. Chem. 278:47707–47712. - PubMed
-
- Ahlen, K., A. Berg, F. Stiger, A. Tengholm, A. Siegbahn, E. Gylfe, R.K. Reed, and K. Rubin. 1998. Cell interactions with collagen matrices in vivo and in vitro depend on phosphatidylinositol 3-kinase and free cytoplasmic calcium. Cell Adhes. Commun. 5:461–473. - PubMed
-
- Anliker, B., and J. Chun. 2004. Lysophospholipid G protein-coupled receptors. J. Biol. Chem. 279:20555–20558. - PubMed
-
- Arber, S., F.A. Barbayannis, H. Hanser, C. Schneider, C.A. Stanyon, O. Bernard, and P. Caroni. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 393:805–809. - PubMed
-
- Bamburg, J.R. 1999. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15:185–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

