Growth inhibition and apoptosis induced by daunomycin-conjugated triplex-forming oligonucleotides targeting the c-myc gene in prostate cancer cells
- PMID: 16449206
- PMCID: PMC1356532
- DOI: 10.1093/nar/gkj473
Growth inhibition and apoptosis induced by daunomycin-conjugated triplex-forming oligonucleotides targeting the c-myc gene in prostate cancer cells
Abstract
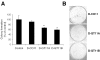
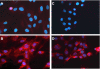
Covalent attachment of intercalating agents to triplex-forming oligonucleotides (TFOs) is a promising strategy to enhance triplex stability and biological activity. We have explored the possibility to use the anticancer drug daunomycin as triplex stabilizing agent. Daunomycin-conjugated TFOs (dauno-TFOs) bind with high affinity and maintain the sequence-specificity required for targeting individual genes in the human genome. Here, we examined the effects of two dauno-TFOs targeting the c-myc gene on gene expression, cell proliferation and survival. The dauno-TFOs were directed to sequences immediately upstream (dauno-GT11A) and downstream (dauno-GT11B) the major transcriptional start site in the c-myc gene. Both dauno-TFOs were able to down-regulate promoter activity and transcription of the endogenous gene. Myc-targeted dauno-TFOs inhibited growth and induced apoptosis of prostate cancer cells constitutively expressing the gene. Daunomycin-conjugated control oligonucleotides with similar sequences had only minimal effects, confirming that the activity of dauno-TFOs was sequence-specific and triplex-mediated. To test the selectivity of dauno-TFOs, we examined their effects on growth of normal human fibroblasts, which express low levels of c-myc. Despite their ability to inhibit c-myc transcription, both dauno-TFOs failed to inhibit growth of normal fibroblasts at concentrations that inhibited growth of prostate cancer cells. In contrast, daunomycin inhibited equally fibroblasts and prostate cancer cells. Thus, daunomycin per se did not contribute to the antiproliferative activity of dauno-TFOs, although it greatly enhanced their ability to form stable triplexes at the target sites and down-regulate c-myc. Our data indicate that dauno-TFOs are attractive gene-targeting agents for development of new cancer therapeutics.
Figures


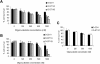

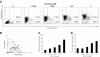

Similar articles
-
DNA binding and antigene activity of a daunomycin-conjugated triplex-forming oligonucleotide targeting the P2 promoter of the human c-myc gene.Nucleic Acids Res. 2004 Apr 30;32(8):2396-410. doi: 10.1093/nar/gkh527. Print 2004. Nucleic Acids Res. 2004. PMID: 15121897 Free PMC article.
-
Improved synthesis of daunomycin conjugates with triplex-forming oligonucleotides. The polypurine tract of HIV-1 as a target.Bioorg Med Chem. 2005 May 2;13(9):3209-18. doi: 10.1016/j.bmc.2005.02.040. Bioorg Med Chem. 2005. PMID: 15809156
-
Inhibition of transcription of the human c-myc protooncogene by intermolecular triplex.Biochemistry. 1998 Feb 24;37(8):2299-304. doi: 10.1021/bi9718191. Biochemistry. 1998. PMID: 9485376
-
The anti-gene strategy: control of gene expression by triplex-forming-oligonucleotides.Anticancer Drug Des. 1991 Dec;6(6):569-84. Anticancer Drug Des. 1991. PMID: 1772570 Review.
-
The proto-oncogene c-myc and apoptosis.Oncogene. 1998 Dec 24;17(25):3351-7. doi: 10.1038/sj.onc.1202592. Oncogene. 1998. PMID: 9916997 Review.
Cited by
-
The dynamics of forming a triplex in an artificial telomere inferred by DNA mechanics.Nucleic Acids Res. 2019 Sep 5;47(15):e86. doi: 10.1093/nar/gkz464. Nucleic Acids Res. 2019. PMID: 31114915 Free PMC article.
-
LncRNA MIR100HG promotes cell proliferation in triple-negative breast cancer through triplex formation with p27 loci.Cell Death Dis. 2018 Jul 24;9(8):805. doi: 10.1038/s41419-018-0869-2. Cell Death Dis. 2018. PMID: 30042378 Free PMC article.
-
Active Substances from the Micro-Immunotherapy Medicine 2LC1® Show In Vitro Anti-Cancer Properties in Colon, Prostate, and Breast Cancer Models and Immune-Enhancing Capabilities in Human Macrophages.Int J Mol Sci. 2025 May 1;26(9):4300. doi: 10.3390/ijms26094300. Int J Mol Sci. 2025. PMID: 40362536 Free PMC article.
-
Sequence-specific targeting of IGF-I and IGF-IR genes by camptothecins.FASEB J. 2010 Jul;24(7):2235-44. doi: 10.1096/fj.09-132324. Epub 2010 Feb 23. FASEB J. 2010. PMID: 20179147 Free PMC article.
-
Triplex-forming oligonucleotides as an anti-gene technique for cancer therapy.Front Pharmacol. 2022 Dec 21;13:1007723. doi: 10.3389/fphar.2022.1007723. eCollection 2022. Front Pharmacol. 2022. PMID: 36618947 Free PMC article. Review.
References
-
- Praseuth D., Guieysse A.L., Helene C. Triple helix formation and the antigene strategy for sequence-specific control of gene expression. Biochim. Biophys. Acta. 1999;1489:181–206. - PubMed
-
- Casey B.P., Glazer P.M. Gene targeting via triple-helix formation. Prog. Nucleic Acid Res. Mol. Biol. 2001;67:163–192. - PubMed
-
- Winters T.A. Gene targeted agents: new opportunities for rational drug development. Curr. Opin. Mol. Ther. 2000;2:670–681. - PubMed
-
- Catapano C.V., McGuffie E.M., Pacheco D., Carbone G.M. Inhibition of gene expression and cell proliferation by triple helix-forming oligonucleotides directed to the c-myc gene. Biochemistry. 2000;39:5126–5138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

